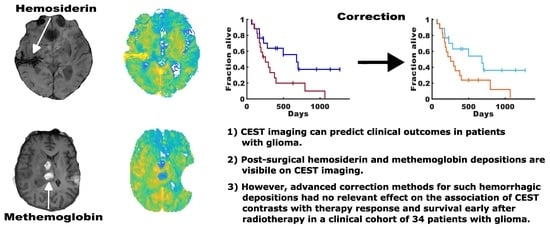
Post-Surgical Depositions of Blood Products Are No Major Confounder for the Diagnostic and Prognostic Performance of CEST MRI in Patients with Glioma
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. CEST Contrast Maps of Participants with Larger Depositions of mHb and Hs
3.2. Differences between the Mean CEST Contrast Values of Uncorrected and Corrected Tumor Volumes
3.3. Association of CEST Contrast Values of Uncorrected and Corrected Tumor Volumes with Therapy Response
3.4. Association of Mean CEST Contrast Values of Uncorrected and Corrected Tumor Volumes with Overall Survival
3.5. Supermedian Analysis of the Mean CEST Contrast Values of Uncorrected and Corrected Tumor Volumes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| mHb | Hs | mHb | CEC | mHb | WTC | Hs | CEC | Hs | WTC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MTRrexAPT | Mean | 0.206 | 0.249 | 0.206 | 0.247 | 0.206 | 0.243 | 0.249 | 0.247 | 0.249 | 0.243 |
| SD | 0.070 | 0.038 | 0.070 | 0.037 | 0.070 | 0.029 | 0.038 | 0.037 | 0.038 | 0.029 | |
| P | 0.035 * | 0.042 | 0.052 * | 0.817 | 0.568 | ||||||
| MTRrexMT | Mean | 0.272 | 0.325 | 0.272 | 0.381 | 0.272 | 0.470 | 0.325 | 0.381 | 0.325 | 0.470 |
| SD | 0.098 | 0.074 | 0.098 | 0.073 | 0.098 | 0.063 | 0.074 | 0.073 | 0.074 | 0.063 | |
| P | 0.134 | <0.001 * | <0.001 * | 0.004 * | <0.001 * | ||||||
| APTwasym | Mean [%] | 0.712 | 1.164 | 0.712 | 1.379 | 0.712 | 0.898 | 1.164 | 1.379 | 1.164 | 0.898 |
| SD [%] | 1.595 | 0.837 | 1.595 | 0.553 | 1.595 | 0.528 | 0.837 | 0.553 | 0.837 | 0.528 | |
| P | 0.436 | 0.200 | 0.313 | 0.527 | 0.013 * | ||||||
| MTconst | Mean | 0.145 | 0.163 | 0.145 | 0.173 | 0.145 | 0.163 | 0.163 | 0.173 | 0.163 | 0.163 |
| SD | 0.050 | 0.036 | 0.050 | 0.028 | 0.050 | 0.023 | 0.036 | 0.028 | 0.036 | 0.023 | |
| P | 0.240 | 0.052 * | 0.156 | 0.174 | 0.915 | ||||||
| Tumor Volume | CE | CEC | WT | WTC | |
|---|---|---|---|---|---|
| MTRRex APT | P | 0.546 | 0.458 | 0.523 | 0.501 |
| AUC | 0.438 | 0.424 | 0.566 | 0.569 | |
| BP sens | 0.500 | 0.500 | 0.688 | 0.688 | |
| BP spez | 0.556 | 0.556 | 0.556 | 0.556 | |
| MTRRex MT | P | 0.081 | 0.044 * | 0.184 | 0.208 |
| AUC | 0.677 | 0.705 | 0.635 | 0.628 | |
| BP sens | 0.556 | 0.667 | 0.500 | 0.500 | |
| BP spez | 0.813 | 0.688 | 0.875 | 0.938 | |
| APTwASYM | P | 0.904 | 0.986 | 0.717 | 0.617 |
| AUC | 0.514 | 0.503 | 0.538 | 0.552 | |
| BP sens | 0.667 | 0.667 | 0.278 | 0.556 | |
| BP spez | 0.625 | 0.563 | 1.000 | 0.625 | |
| MTconst | P | 0.001 * | 0.002 * | <0.001 * | <0.001 * |
| AUC | 0.826 | 0.816 | 0.868 | 0.861 | |
| BP sens | 0.667 | 0.722 | 0.722 | 0.722 | |
| BP spez | 1.000 | 0.938 | 0.938 | 0.938 | |
| Participants (n) | 34 | ||||
| Tumor Volume | CE | CEC | WT | WTC | |
|---|---|---|---|---|---|
| MTRRexAPT | P | 0.056 | 0.110 | 0.417 | 0.417 |
| HR | 2.439 | 2.110 | 1.526 | 1.525 | |
| OS+ | 225 | 253 | 225 | 225 | |
| OS− | 416 | 392 | 392 | 392 | |
| MTRRexMT | P | 0.919 | 0.964 | 0.559 | 0.847 |
| HR | 0.958 | 1.068 | 1.389 | 1.179 | |
| OS+ | 315 | 315 | 294 | 315 | |
| OS− | 280 | 280 | 280 | 225 | |
| APTwasym | P | 0.040 * | 0.095 | 0.084 | 0.020 * |
| HR | 2.634 | 2.240 | 2.304 | 2.990 | |
| OS+ | 215 | 215 | 225 | 215 | |
| OS− | 392 | 392 | 398 | 398 | |
| MTconst | P | 0.068 | 0.068 | 0.044 * | 0.044 * |
| HR | 2.330 | 2.330 | 2.536 | 2.536 | |
| OS+ | 228 | 228 | 215 | 215 | |
| OS− | 315 | 315 | 392 | 392 | |
| Participants (n) | 34 | ||||
References
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol. Imaging 2018, 2018, 6828396. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, O.M.; Álvarez-Torres, M.D.M.; Figueiredo, P.; Hangel, G.; Keil, V.C.; Nechifor, R.E.; Riemer, F.; Schmainda, K.M.; Warnert, E.A.H.; Wiegers, E.C.; et al. High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 1: Perfusion and Diffusion Techniques. Front. Oncol. 2022, 12, 810263. [Google Scholar] [CrossRef] [PubMed]
- Booth, T.C.; Wiegers, E.C.; Warnert, E.A.H.; Schmainda, K.M.; Riemer, F.; Nechifor, R.E.; Keil, V.C.; Hangel, G.; Figueiredo, P.; Álvarez-Torres, M.D.M.; et al. High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 2: Spectroscopy, Chemical Exchange Saturation, Multiparametric Imaging, and Radiomics. Front. Oncol. 2021, 11, 811425. [Google Scholar]
- van Zijl, P.C.M.; Lam, W.W.; Xu, J.; Knutsson, L.; Stanisz, G.J. Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum. NeuroImage 2018, 168, 222–241. [Google Scholar]
- Doeberitz, N.v.K.; Kroh, F.; Breitling, J.; König, L.; Maksimovic, S.; Graß, S.; Adeberg, S.; Scherer, M.; Unterberg, A.; Bendszus, M.; et al. CEST imaging of the APT and ssMT predict the overall survival of patients with glioma at the first follow-up after completion of radiotherapy at 3T. Radiother. Oncol. 2023, 184, 109694. [Google Scholar] [CrossRef]
- Mehrabian, H.; Myrehaug, S.; Soliman, H.; Sahgal, A.; Stanisz, G.J. Evaluation of Glioblastoma Response to Therapy with Chemical Exchange Saturation Transfer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Paech, D.; Dreher, C.; Regnery, S.; Meissner, J.-E.; Goerke, S.; Windschuh, J.; Oberhollenzer, J.; Schultheiss, M.; Deike-Hofmann, K.; Bickelhaupt, S.; et al. Relaxation-compensated amide proton transfer (APT) MRI signal intensity is associated with survival and progression in high-grade glioma patients. Eur. Radiol. 2019, 29, 4957–4967. [Google Scholar] [CrossRef] [PubMed]
- Regnery, S.; Adeberg, S.; Dreher, C.; Oberhollenzer, J.; Meissner, J.-E.; Goerke, S.; Windschuh, J.; Deike-Hofmann, K.; Bickelhaupt, S.; Zaiss, M.; et al. Chemical exchange saturation transfer MRI serves as predictor of early progression in glioblastoma patients. Oncotarget 2018, 9, 28772–28783. [Google Scholar] [CrossRef]
- Chan, R.W.; Chen, H.; Myrehaug, S.; Atenafu, E.G.; Stanisz, G.J.; Stewart, J.; Maralani, P.J.; Chan, A.K.M.; Daghighi, S.; Ruschin, M.; et al. Quantitative CEST and MT at 1.5T for monitoring treatment response in glioblastoma: Early and late tumor progression during chemoradiation. J. Neurooncol. 2021, 151, 267–278. [Google Scholar]
- Kroh, F.; Doeberitz, N.V.K.; Breitling, J.; Maksimovic, S.; König, L.; Adeberg, S.; Scherer, M.; Unterberg, A.; Bendszus, M.; Wick, W.; et al. Semi-solid MT and APTw CEST-MRI predict clinical outcome of patients with glioma early after radiotherapy. Magn. Reson. Med. 2023, 90, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Meissner, J.; Korzowski, A.; Regnery, S.; Goerke, S.; Breitling, J.; Floca, R.O.; Debus, J.; Schlemmer, H.; Ladd, M.E.; Bachert, P.; et al. Early response assessment of glioma patients to definitive chemoradiotherapy using chemical exchange saturation transfer imaging at 7 T. J. Magn. Reson. Imaging 2019, 50, 1268–1277. [Google Scholar] [CrossRef]
- Ma, B.; Blakeley, J.O.; Hong, X.; Zhang, H.; Jiang, S.; Blair, L.; Zhang, Y.; Heo, H.-Y.; Zhang, M.; van Zijl, P.C.M.; et al. Applying amide proton transfer-weighted MRI to distinguish pseudoprogression from true progression in malignant gliomas. J. Magn. Reson. Imaging 2016, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Chen, Y.; Lv, X.; Lv, Y.; Zhou, J.; Xi, S.; Dou, W.; Qian, L.; Zheng, H.; et al. Diagnostic performance of multiparametric MRI in the evaluation of treatment response in glioma patients at 3T. J. Magn. Reson. Imaging 2020, 51, 1154–1161. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.S.; Park, K.J.; Kim, S.J.; Kim, J.H.; Smith, S.A. Pre- and Posttreatment Glioma: Comparison of Amide Proton Transfer Imaging with MR Spectroscopy for Biomarkers of Tumor Proliferation. Radiology 2016, 278, 514–523. [Google Scholar] [CrossRef]
- Park, K.J.; Kim, H.S.; Park, J.E.; Shim, W.H.; Kim, S.J.; Smith, S.A. Added value of amide proton transfer imaging to conventional and perfusion MR imaging for evaluating the treatment response of newly diagnosed glioblastoma. Eur. Radiol. 2016, 26, 4390–4403. [Google Scholar] [CrossRef]
- Park, Y.W.; Ahn, S.S.; Kim, E.H.; Kang, S.-G.; Chang, J.H.; Kim, S.H.; Zhou, J.; Lee, S.-K. Differentiation of recurrent diffuse glioma from treatment-induced change using amide proton transfer imaging: Incremental value to diffusion and perfusion parameters. Neuroradiology 2021, 63, 363–372. [Google Scholar] [CrossRef]
- Sanghvi, D. Post-treatment imaging of high-grade gliomas. Indian J. Radiol. Imaging 2015, 25, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, R.; Ueda, J.; Saito, S. Quantitative Susceptibility Mapping and Amide Proton Transfer-Chemical Exchange Saturation Transfer for the Evaluation of Intracerebral Hemorrhage Model. Int. J. Mol. Sci. 2023, 24, 6627. [Google Scholar] [CrossRef]
- Wang, M.; Hong, X.; Chang, C.-F.; Li, Q.; Ma, B.; Zhang, H.; Xiang, S.; Heo, H.-Y.; Zhang, Y.; Lee, D.-H.; et al. Simultaneous detection and separation of hyperacute intracerebral hemorrhage and cerebral ischemia using amide proton transfer MRI. Magn. Reson. Med. 2015, 74, 42–50. [Google Scholar]
- Lai, J.H.; Liu, J.; Yang, T.; Huang, J.; Liu, Y.; Chen, Z.; Lee, Y.; Leung, G.K.; Chan, K.W. Chemical Exchange Saturation Transfer Magnetic Resonance Imaging for Longitudinal Assessment of Intracerebral Hemorrhage and Deferoxamine Treatment at 3T in a Mouse Model. Stroke 2023, 54, 255–264. [Google Scholar] [CrossRef]
- Zaiss, M.; Windschuh, J.; Paech, D.; Meissner, J.-E.; Burth, S.; Schmitt, B.; Kickingereder, P.; Wiestler, B.; Wick, W.; Bendszus, M.; et al. Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. NeuroImage 2015, 112, 180–188. [Google Scholar] [CrossRef]
- Zhou, J.; Zaiss, M.; Knutsson, L.; Sun, P.Z.; Ahn, S.S.; Aime, S.; Bachert, P.; Blakeley, J.O.; Cai, K.; Chappell, M.A.; et al. Review and consensus recommendations on clinical APT-weighted imaging approaches at 3T: Application to brain tumors. Magn. Reson. Med. 2022, 88, 546–574. [Google Scholar] [PubMed]
- Zhou, J.; Heo, H.-Y.; Knutsson, L.; van Zijl, P.C.; Jiang, S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J. Magn. Reson. Imaging 2019, 50, 347–364. [Google Scholar] [PubMed]
- Goerke, S.; Soehngen, Y.; Deshmane, A.; Zaiss, M.; Breitling, J.; Boyd, P.S.; Herz, K.; Zimmermann, F.; Klika, K.D.; Schlemmer, H.P.; et al. Relaxation-compensated APT and rNOE CEST-MRI of human brain tumors at 3 T. Magn. Reson. Med. 2019, 82, 622–632. [Google Scholar] [CrossRef]
- Wen, P.Y.; Chang, S.M.; Bent, M.J.V.D.; Vogelbaum, M.A.; Macdonald, D.R.; Lee, E.Q. Response assessment in neuro-oncology clinical trials. J. Clin. Oncol. 2017, 35, 2439. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [PubMed]
- Eisenstat, D.D.; Pollack, I.F.; Demers, A.; Sapp, M.V.; Lambert, P.; Weisfeld-Adams, J.D.; Burger, P.C.; Gilles, F.; Davis, R.L.; Packer, R.; et al. Impact of tumor location and pathological discordance on survival of children with midline high-grade gliomas treated on Children’s Cancer Group high-grade glioma study CCG-945. J. Neurooncol. 2015, 121, 573–581. [Google Scholar]
- Deshmane, A.; Zaiss, M.; Lindig, T.; Herz, K.; Schuppert, M.; Gandhi, C.; Bender, B.; Ernemann, U.; Scheffler, K. 3D gradient echo snapshot CEST MRI with low power saturation for human studies at 3T. Magn. Reason. Med. 2019, 81, 2412–2423. [Google Scholar] [CrossRef]
- Zaiss, M.; Ehses, P.; Scheffler, K. Snapshot-CEST: Optimizing spiral-centric-reordered gradient echo acquisition for fast and robust 3D CEST MRI at 9.4 T. NMR Biomed. 2018, 31, e3879. [Google Scholar] [CrossRef]
- Schuenke, P.; Windschuh, J.; Roeloffs, V.; Ladd, M.E.; Bachert, P.; Zaiss, M. Simultaneous mapping of water shift and B1 (WASABI)-Application to field-Inhomogeneity correction of CEST MRI data. Magn. Reson. Med. 2017, 77, 571–580. [Google Scholar] [CrossRef]
- Breitling, J.; Korzowski, A.; Kempa, N.; Boyd, P.S.; Paech, D.; Schlemmer, H.; Ladd, M.E.; Bachert, P.; Goerke, S. Motion correction for three-dimensional chemical exchange saturation transfer imaging without direct water saturation artifacts. NMR Biomed. 2022, 35, e4720. [Google Scholar] [CrossRef]
- Windschuh, J.; Zaiss, M.; Meissner, J.-E.; Paech, D.; Radbruch, A.; Ladd, M.E.; Bachert, P. Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR Biomed. 2015, 28, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Bradley, W.G., Jr. MR appearance of hemorrhage in the brain. Radiology 1993, 189, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; van der Bom, I.M.J.; Zu, Z.; Lin, G.; Zhao, Y.; Gounis, M.J. Chemical exchange saturation transfer effect in blood. Magn. Reson. Med. 2014, 71, 1082–1092. [Google Scholar] [CrossRef]
- Shah, S.M.; Mougin, O.E.; Carradus, A.J.; Geades, N.; Dury, R.; Morley, W.; Gowland, P.A. The z-spectrum from human blood at 7T. NeuroImage 2018, 167, 31–40. [Google Scholar] [CrossRef] [PubMed]








| Characteristic | Number (n) | Percentage | |
|---|---|---|---|
| Age at diagnosis | Mean 59.2 ± 15.6 | 34 | |
| Therapy response at the 1st FU 1 | Stable disease (SD) | 16 | 47.1% |
| Progressive disease (PD) | 18 | 52.9% | |
| Overall survival | Median 287 days (min. 63, max. 1271) | ||
| Alive at data cut-off | 10 | 29.4% | |
| Sex | Male | 19 | 55.9% |
| Female | 15 | 44.1% | |
| Treatment for | Initial disease | 31 | 91.2% |
| Progressive disease | 3 | 8.8% | |
| Therapy | Radiation | 6 | 17.6% |
| Chemoradiation | 28 | 82.4% | |
| Debulking surgery | 21 | 61.8% | |
| Diagnosis | GBM 2 | 28 | 82.4% |
| Gliosarcoma | 2 | 5.9% | |
| Astrocytoma | 4 | 11.8% | |
| WHO 3 | II | 1 | 2.9% |
| III | 1 | 2.9% | |
| IV | 32 | 94.1% | |
| IDH 4 status | IDHwt 5 | 28 | 82.4% |
| IDHmut 6 | 4 | 11.8% | |
| n/a | 2 | 5.9% | |
| MGMT promotor methylation | Yes | 19 | 55.9% |
| No | 12 | 35.3% | |
| n/a | 3 | 8.8% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Knebel Doeberitz, N.; Kroh, F.; König, L.; Boyd, P.S.; Graß, S.; Bauspieß, C.; Scherer, M.; Unterberg, A.; Bendszus, M.; Wick, W.; et al. Post-Surgical Depositions of Blood Products Are No Major Confounder for the Diagnostic and Prognostic Performance of CEST MRI in Patients with Glioma. Biomedicines 2023, 11, 2348. https://doi.org/10.3390/biomedicines11092348
von Knebel Doeberitz N, Kroh F, König L, Boyd PS, Graß S, Bauspieß C, Scherer M, Unterberg A, Bendszus M, Wick W, et al. Post-Surgical Depositions of Blood Products Are No Major Confounder for the Diagnostic and Prognostic Performance of CEST MRI in Patients with Glioma. Biomedicines. 2023; 11(9):2348. https://doi.org/10.3390/biomedicines11092348
Chicago/Turabian Stylevon Knebel Doeberitz, Nikolaus, Florian Kroh, Laila König, Philip S. Boyd, Svenja Graß, Cora Bauspieß, Moritz Scherer, Andreas Unterberg, Martin Bendszus, Wolfgang Wick, and et al. 2023. "Post-Surgical Depositions of Blood Products Are No Major Confounder for the Diagnostic and Prognostic Performance of CEST MRI in Patients with Glioma" Biomedicines 11, no. 9: 2348. https://doi.org/10.3390/biomedicines11092348
APA Stylevon Knebel Doeberitz, N., Kroh, F., König, L., Boyd, P. S., Graß, S., Bauspieß, C., Scherer, M., Unterberg, A., Bendszus, M., Wick, W., Bachert, P., Debus, J., Ladd, M. E., Schlemmer, H. -P., Goerke, S., Korzowski, A., & Paech, D. (2023). Post-Surgical Depositions of Blood Products Are No Major Confounder for the Diagnostic and Prognostic Performance of CEST MRI in Patients with Glioma. Biomedicines, 11(9), 2348. https://doi.org/10.3390/biomedicines11092348