A Comprehensive Review on Neuroimmunology: Insights from Multiple Sclerosis to Future Therapeutic Developments
Abstract
:1. Introduction
1.1. Background
1.2. Purpose and Scope of the Review
1.3. Methodology of Review Selection
- (a)
- Search Strategy: An extensive literature search was performed using multiple electronic databases such as PubMed, Scopus, Web of Science, and Google Scholar. Our search strategy comprised pertinent keywords and phrases related to neuroimmunology, multiple sclerosis, neuroinflammation, immune system dysfunction, central nervous system therapies, as well as therapeutic interventions with no restrictions placed on publication dates, allowing a broad representation.
- (b)
- Inclusion and Exclusion Criteria:
2. The Basics of Neuroimmunology
2.1. Definition and Overview
2.2. Key Players in Neuroimmunology: Cells and Molecules
2.3. Neuroimmune Communication Pathways
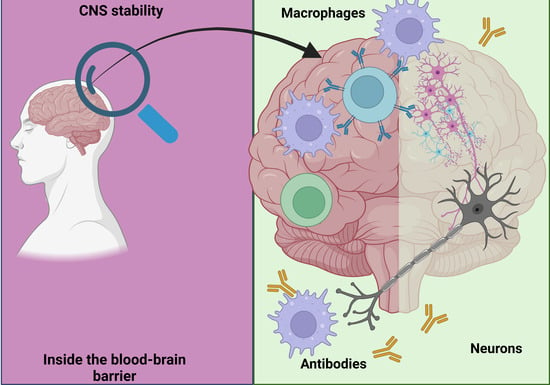
2.4. The Role of the Immune System in Maintaining Neuronal Health
3. The Neuroimmunology of Multiple Sclerosis
3.1. Pathophysiology of MS: Neuroimmunological Perspective
3.2. The Role of the Immune System in MS
3.2.1. The Blood–Brain Barrier
3.2.2. The Role of T Cells
3.2.3. The Role of B Cells
3.2.4. The Role of NK Cells
4. Immune Mechanisms in Neurological Disorders
4.1. Immune-Related Neurological Disorders: MS and Beyond
4.2. The Role of Neuroimmunity in the Pathogenesis of Neurological Disorders
5. Clinical Neuroimmunology Research
5.1. Recent Advancements in Clinical Research of Neuroimmunological Disorders
5.2. Methodologies Used in Clinical Neuroimmunology Research
5.3. Challenges and Opportunities in Current Research
5.4. Case Studies: Application of Clinical Research in Managing MS and Other Neurological Disorders
6. Neuroimmunological Aspects of Health and Diseases
6.1. The Impact of Neuroimmunology on General Health
6.2. The Role of Neuroimmunology in Non-Neurological Diseases
6.3. Neuroimmunological Perspectives in Chronic Diseases
6.4. The Influence of Environmental and Lifestyle Factors on Neuroimmune Health
7. Developments in the Immunotherapy of Neurological Disorders
7.1. Current Approaches to Immunotherapy in Neurological Disorders
7.2. Advances in MS Immunotherapy
7.3. Challenges in Developing Effective Immunotherapies
7.4. Future Directions in Immunotherapeutic Strategies for Neurological Disorders
8. Emerging Fields in Neuroimmunology
8.1. Neuroimmunology in Aging and Neurodegenerative Disorders
8.2. Pediatric Neuroimmunology
8.3. Neuroimmunoendocrinology: The Interactions between the Nervous, Immune, and Endocrine Systems
8.4. The Role of Microbiota in Neuroimmunology
9. Conclusions
9.1. Summary of Key Findings
- (a)
- Immune Dysregulation: Multiple sclerosis is characterized by autoreactive T cells attacking the central nervous system, leading to inflammation and nerve fiber demyelination. Understanding their activation and regulation is crucial to discovering potential therapeutic targets for treatment;
- (b)
- Cells Play an Important Role in Multiple Sclerosis Pathogenesis: B cells have emerged as key contributors to MS pathogenesis. By producing autoantibodies and modulating T-cell responses, these B cells play an integral part in tissue damage and neuroinflammation. Depletion therapies targeting B cells have shown promise as a possible treatment option for MS;
- (c)
- Genetic Susceptibility: Genome-wide association studies have linked various genetic variations with MS susceptibility, enabling personalized therapy approaches and insights into genetic factors contributing to its cause;
- (d)
- Environmental Triggers: Research has shed light on how environmental triggers like smoking, vitamin D deficiency, and viral infections interact to increase MS risk and severity. Researchers have also investigated the interaction between genetic predisposition and environmental influences in driving this process forward;
- (e)
- Neuroprotective Strategies: Because MS leads to neurodegeneration, researchers are investigating neuroprotective measures that preserve nerve function and facilitate repair processes. Researching molecules involved with remyelination and axonal support could reveal therapeutic targets;
- (f)
- Immunomodulatory Medications: Immune-system-targeted disease-modifying therapies have become a cornerstone of MS treatment, demonstrating significant slowing in disease progression and enhanced patient outcomes through early use.
- (g)
- Studies have shed light on how gut microbiota influences immune responses and possibly contributes to MS. Understanding this relationship could lead to novel treatment approaches aimed at altering gut bacteria composition and functioning;
- (h)
- Neuroimmunological studies have provided valuable insights into multiple sclerosis by shedding light on its gut–brain axis, genetic predispositions, environmental influences, B-cell involvement, immune dysregulation, and neuroprotective treatments as key elements. These discoveries hold great promise for developing more targeted and effective treatment strategies and ultimately improving the quality of life among those living with this complex neurological disorder.
9.2. Implications for Future Research
- (a)
- Uncovering Underlying Mechanisms: Exploring the interrelations between MS’s immune and neurological systems can provide insight into specific physiological and molecular processes driving disease development while pinpointing key immune cell types, cytokines, and chemokines associated with inflammation and demyelination, which will allow targeted therapies;
- (b)
- Neuroprotective Strategies: Although immune dysregulation is currently at the core of MS treatments, future research should prioritize neuroprotective approaches that preserve and restore damaged nerve cells. Deliberate identification of chemicals or pathways that promote neuronal survival and remyelination is key for slowing disability progression;
- (c)
- Microbiome and Gut–Brain Axis: Understanding the role of gut microbiomes and gut–brain axis in MS pathophysiology can lead to innovative treatments that control immune responses and decrease disease activity, including interventions that regulate gut microbiomes;
- (d)
- Precision Medicine: Advancements in molecular profiling, biomarker discovery, and tailored treatment strategies hold great potential to optimize medication selection treatment efficacy and minimize side effects in MS patients;
- (e)
- Immunological Tolerance and Immunomodulation: Generating novel immunological tolerance mechanisms against myelin antigens could revolutionize MS therapy. Investigating innovative immunomodulatory strategies such as antigen-specific therapy or immune cell modulation could slow disease progression;
- (f)
- Innovative Imaging Technologies: State-of-the-art imaging techniques like optical coherence tomography and advanced MRI methods offer more precise and early diagnostic indicators, monitor disease progression, and assess treatment efficacy in real time;
- (g)
- Drug Repurposing: Investigating the therapeutic potential of existing neuroprotective or immunomodulatory medications can hasten the discovery of new MS treatments.
9.3. Final Thoughts
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nutma, E.; Willison, H.; Martino, G.; Amor, S. Neuroimmunology—The past, present and future. Clin. Exp. Immunol. 2019, 197, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Pachner, A.R. The Neuroimmunology of Multiple Sclerosis: Fictions and Facts. Front. Neurol. 2021, 12, 796378. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, L.J.; Subramaniam, S.; MacKenzie, G.; Shah, D.K. Maximising the potential of neuroimmunology. Brain Behav. Immun. 2020, 87, 189–192. [Google Scholar] [CrossRef]
- Multiple Sclerosis. National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/health-information/disorders/multiple-sclerosis (accessed on 24 July 2023).
- Kraus, A.; Buckley, K.M.; Salinas, I. Sensing the world and its dangers: An evolutionary perspective in neuroimmunology. eLife 2021, 10, e66706. [Google Scholar] [CrossRef]
- Aguilera-Lizarraga, J.; Florens, M.V.; Viola, M.F.; Jain, P.; Decraecker, L.; Appeltans, I.; Cuende-Estevez, M.; Fabre, N.; Van Beek, K.; Perna, E.; et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 2021, 590, 151–156. [Google Scholar] [CrossRef]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.-R.; Bean, B.P.; Woolf, C.J.; et al. Nociceptors Are Interleukin-1β Sensors. J. Neurosci. 2008, 28, 14062–14073. [Google Scholar] [CrossRef]
- Nagashima, H.; Mahlakõiv, T.; Shih, H.Y.; Davis, F.P.; Meylan, F.; Huang, Y.; Harrison, O.J.; Yao, C.; Mikami, Y.; Urban, J.F., Jr.; et al. Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation. Immunity 2019, 51, 682–695.e6. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Pachnis, V. Neuroimmune regulation during intestinal development and homeostasis. Nat. Immunol. 2017, 18, 116–122. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Neuroimmune communication. Nat. Immunol. 2017, 18, 115. [CrossRef] [PubMed]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.; Sorkin, E.; Keller, M.; Müller, J. Changes in blood hormone levels during the immune response. Proc. Soc. Exp. Biol. Med. 1975, 150, 466–470. [Google Scholar] [CrossRef]
- Besedovsky, H.; del Rey, A.; Sorkin, E.; Dinarello, C.A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 1986, 233, 652–654. [Google Scholar] [CrossRef]
- Pace, T.W.W.; Hu, F.; Miller, A.H. Cytokine-Effects on Glucocorticoid Receptor Function: Relevance to Glucocorticoid Resistance and the Pathophysiology and Treatment of Major Depression. Brain Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Engelhardt, B. The blood-central nervous system barriers actively control immune cell entry into the central nervous system. Curr. Pharm. Des. 2008, 14, 1555–1565. [Google Scholar] [CrossRef]
- Bechmann, I.; Galea, I.; Perry, V.H. What is the blood-brain barrier (not)? Trends Immunol. 2007, 28, 5–11. [Google Scholar] [CrossRef]
- Dolei, A.; Garson, J.A.; Arru, G.; Clerici, M.; Germi, R.; Marche, P.N.; Perron, H. Multiple sclerosis-associated retrovirus and related human endogenous retrovirus-W in patients with multiple sclerosis. J. Neuroimmunol. 2014, 266, 87–88. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.; Panitch, H.; Bar-Or, A.; Dunn, J.; Freedman, M.S.; Gazda, S.; Campagnolo, D.; Deutsch, F.; Arnold, D. Glatiramer acetate after induction therapy with mitoxantrone in relapsing multiple sclerosis. Mult. Scler. 2008, 14, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Høglund, R.A.; Maghazachi, A.A. Multiple sclerosis and the role of immune cells. World J. Exp. Med. 2014, 4, 27–37. [Google Scholar] [CrossRef]
- Glatigny, S.; Bettelli, E. Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS). Cold Spring Harb. Perspect. Med. 2018, 8, a028977. [Google Scholar] [CrossRef]
- Procaccini, C.; De Rosa, V.; Pucino, V.; Formisano, L.; Matarese, G. Animal models of Multiple Sclerosis. Eur. J. Pharmacol. 2015, 759, 182–191. [Google Scholar] [CrossRef]
- van Noort, J.M.; Verbeek, R.; Meilof, J.F.; Polman, C.H.; Amor, S. Autoantibodies against alpha B-crystallin, a candidate autoantigen in multiple sclerosis, are part of a normal human immune repertoire. Mult. Scler. 2006, 12, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Mathey, E.K.; Derfuss, T.; Storch, M.K.; Williams, K.R.; Hales, K.; Woolley, D.R.; Al-Hayani, A.; Davies, S.N.; Rasband, M.N.; Olsson, T.; et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J. Exp. Med. 2007, 204, 2363–2372. [Google Scholar] [CrossRef]
- Öberg, L.; Johansson, S.; Michaëlsson, J.; Tomasello, E.; Vivier, E.; Kärre, K.; Höglund, P. Loss or mismatch of MHC class I is sufficient to trigger NK cell-mediated rejection of resting lymphocytes in vivo—Role of KARAP/DAP12-dependent and -independent pathways. Eur. J. Immunol. 2004, 34, 1646–1653. [Google Scholar] [CrossRef]
- Cella, M.; Fuchs, A.; Vermi, W.; Facchetti, F.; Otero, K.; Lennerz, J.K.M.; Doherty, J.M.; Mills, J.C.; Colonna, M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009, 457, 722–725. [Google Scholar] [CrossRef]
- Al-Ani, M.; Elemam, N.M.; Hundt, J.E.; Maghazachi, A.A. Drugs for Multiple Sclerosis Activate Natural Killer Cells: Do They Protect Against COVID-19 Infection? Infect. Drug Resist. 2020, 13, 3243–3254. [Google Scholar] [CrossRef]
- Maghazachi, A.A. On The Role of Natural Killer Cells in Neurodegenerative Diseases. Toxins 2013, 5, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Zuroff, L.R.; Benjamins, J.A.; Bar-Or, A.; Lisak, R.P. Inflammatory mechanisms underlying cortical injury in progressive multiple sclerosis. Neuroimmunol. Neuroinflamm. 2021, 8, 111. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.P.; Lebos, A.L.; Yao, Y.; Stice, S.L. Immune Response in Neurological Pathology: Emerging Role of Central and Peripheral Immune Crosstalk. Front. Immunol. 2021, 12, 676621. [Google Scholar] [CrossRef]
- Geloso, M.C.; Corvino, V.; Marchese, E.; Serrano, A.; Michetti, F.; D’Ambrosi, N. The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches. Front. Aging Neurosci. 2017, 9, 242. [Google Scholar] [CrossRef]
- Khaw, Y.M.; Cunningham, C.; Tierney, A.; Sivaguru, M.; Inoue, M. Neutrophil-selective deletion of Cxcr2 protects against CNS neurodegeneration in a mouse model of multiple sclerosis. J. Neuroinflamm. 2020, 17, 49. [Google Scholar] [CrossRef]
- Hemond, C.C.; Bakshi, R. Magnetic Resonance Imaging in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028969. [Google Scholar] [CrossRef]
- Qiu, J.G.; Mei, X.L.; Chen, Z.S.; Shi, Z. Cytokine detection by flow cytometry. Methods Mol. Biol. 2014, 1172, 235–242. [Google Scholar] [CrossRef]
- Mahajan, K.R.; Ontaneda, D. The Role of Advanced Magnetic Resonance Imaging Techniques in Multiple Sclerosis Clinical Trials. Neurotherapeutics 2017, 14, 905–923. [Google Scholar] [CrossRef]
- Misra, M.K.; Damotte, V.; Hollenbach, J.A. The immunogenetics of neurological disease. Immunology 2018, 153, 399–414. [Google Scholar] [CrossRef]
- Minen, M.T.; Law, E.F.; Harriott, A.; Seng, E.K.; Hranilovich, J.; Szperka, C.L.; Wells, R.E. Challenges to successful research careers in neurology. Neurology 2020, 95, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Piehl, F. Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis. J. Intern. Med. 2021, 289, 771–791. [Google Scholar] [CrossRef]
- Hanif, N.; Anwer, F. Rituximab. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK564374/ (accessed on 25 July 2023).
- Neuromyelitis Optica. National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/health-information/disorders/neuromyelitis-optica (accessed on 25 July 2023).
- Benziger, C.P.; Roth, G.A.; Moran, A.E. The Global Burden of Disease Study and the Preventable Burden of NCD. Glob. Heart 2016, 11, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Gidron, Y.; Deschepper, R.; De Couck, M.; Thayer, J.F.; Velkeniers, B. The Vagus Nerve Can Predict and Possibly Modulate Non-Communicable Chronic Diseases: Introducing a Neuroimmunological Paradigm to Public Health. J. Clin. Med. 2018, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, G.; Subramaniam, S.; Caldwell, L.J.; Fitzgerald, D.; A Harrison, N.; Hong, S.; Irani, S.R.; Khandaker, G.M.; Liston, A.; E Miron, V.; et al. Research priorities for neuroimmunology: Identifying the key research questions to be addressed by 2030. Wellcome Open Res. 2021, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Medawar, P.B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948, 29, 58–69. [Google Scholar] [PubMed]
- Barker, C.F.; Billingham, R.E. Immunologically privileged sites. Adv. Immunol. 1977, 25, 1–54. [Google Scholar]
- Cserr, H.F.; Knopf, P.M. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: A new view. Immunol. Today 1992, 13, 507–512. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kubotsu, K.; Kida, M.; Kondo, K.; Matsuura, S.; Uchiyama, S.; Yonekawa, O.; Kanno, T. Automated homogeneous liposome-based assay system for total complement activity. Clin. Chem. 1995, 41, 586–590. [Google Scholar] [CrossRef]
- Becher, B.; Prat, A.; Antel, J.P. Brain-immune connection: Immuno-regulatory properties of CNS-resident cells. Glia 2000, 29, 293–304. [Google Scholar] [CrossRef]
- Correale, J.; Mix, E.; Olsson, T.; Kostulas, V.; Fredrikson, S.; Hojeberg, B.; Link, H. CD5+ B-cells and CD4-8-T-cells in neuroimmunological diseases. J. Neuroimmunol. 1991, 32, 123–132. [Google Scholar] [CrossRef]
- Karim, M.; Wang, Y.F. The Study Progress of B Cells and Neuroimmunological Diseases. J. Neurol. Neurophysiol. 2016, 7, 369. [Google Scholar] [CrossRef]
- Gate, D.; Saligrama, N.; Leventhal, O.; Yang, A.C.; Unger, M.S.; Middeldorp, J.; Chen, K.; Lehallier, B.; Channappa, D.; De Los Santos, M.B.; et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 2020, 577, 399–404. [Google Scholar] [CrossRef]
- Togo, T.; Akiyama, H.; Iseki, E.; Kondo, H.; Ikeda, K.; Kato, M.; Oda, T.; Tsuchiya, K.; Kosaka, K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 2002, 124, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Pilch, K.S.; Spaeth, P.J.; Yuki, N.; Wakerley, B.R. Therapeutic complement inhibition: A promising approach for treatment of neuroimmunological diseases. Expert. Rev. Neurother. 2017, 17, 579–591. [Google Scholar] [CrossRef]
- Winkelmann, A.; Loebermann, M.; Barnett, M.; Hartung, H.P.; Zettl, U.K. Vaccination and immunotherapies in neuroimmunological diseases. Nat. Rev. Neurol. 2022, 18, 289–306. [Google Scholar] [CrossRef]
- Brauchle, F.; Rapp, D.; Senel, M.; Huss, A.; Dreyhaupt, J.; Klose, V.; Süße, M.; Stürner, K.H.; Leypoldt, F.; Tumani, H.; et al. Clinical associations and characteristics of the polyspecific intrathecal immune response in elderly patients with non-multiple sclerosis chronic autoimmune-inflammatory neurological diseases—A retrospective cross-sectional study. Front. Neurol. 2023, 14, 1193015. Available online: https://www.frontiersin.org/articles/10.3389/fneur.2023.1193015 (accessed on 25 July 2023). [CrossRef] [PubMed]
- Brenhouse, H.C.; Schwarz, J.M. Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci. Biobehav. Rev. 2016, 70, 288–299. [Google Scholar] [CrossRef]
- Lucerne, K.E.; Osman, A.; Meckel, K.R.; Kiraly, D.D. Contributions of neuroimmune and gut-brain signaling to vulnerability of developing substance use disorders. Neuropharmacology 2021, 192, 108598. [Google Scholar] [CrossRef] [PubMed]
- Charabati, M.; Wheeler, M.A.; Weiner, H.L.; Quintana, F.J. Multiple sclerosis: Neuroimmune crosstalk and therapeutic targeting. Cell 2023, 186, 1309–1327. [Google Scholar] [CrossRef]
- Svokos, K.A.; Salhia, B.; Toms, S.A. Molecular Biology of Brain Metastasis. Int. J. Mol. Sci. 2014, 15, 9519–9530. [Google Scholar] [CrossRef]
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef]
- Villoslada, P.; Moreno, B.; Melero, I.; Pablos, J.L.; Martino, G.; Uccelli, A.; Montalban, X.; Avila, J.; Rivest, S.; Acarin, L.; et al. Immunotherapy for neurological diseases. Clin. Immunol. 2008, 128, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.L. Multiple Sclerosis: New Approaches to Immunotherapy. Neuroscientist 1996, 2, 127–136. [Google Scholar] [CrossRef]
- Hemmer, B.; Nessler, S.; Zhou, D.; Kieseier, B.; Hartung, H.P. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat. Rev. Neurol. 2006, 2, 201–211. [Google Scholar] [CrossRef]
- Kammona, O.; Kiparissides, C. Recent Advances in Antigen-Specific Immunotherapies for the Treatment of Multiple Sclerosis. Brain Sci. 2020, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, R.; Wekerle, H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: From pipe dreams to (therapeutic) pipelines. Proc. Natl. Acad. Sci. USA 2004, 101, 14599–14606. [Google Scholar] [CrossRef]
- Brain Sciences | Free Full-Text | Novel Approaches in the Immunotherapy of Multiple Sclerosis: Cyclization of Myelin Epitope Peptides and Conjugation with Mannan. Available online: https://www.mdpi.com/2076-3425/11/12/1583 (accessed on 25 July 2023).
- Lulu, S.; Waubant, E. Humoral-Targeted Immunotherapies in Multiple Sclerosis. Neurotherapeutics 2013, 10, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Karussis, D. Immunotherapy of Multiple Sclerosis. BioDrugs 2013, 27, 113–148. [Google Scholar] [CrossRef]
- Weiner, H.L. The challenge of multiple sclerosis: How do we cure a chronic heterogeneous disease? Ann. Neurol. 2009, 65, 239–248. [Google Scholar] [CrossRef]
- Hauser, S.L.; Chan, J.R.; Oksenberg, J.R. Multiple sclerosis: Prospects and promise. Ann. Neurol. 2013, 74, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Stürzebecher, C.S.; McFarland, H.F. Immunotherapy of multiple sclerosis: Where are we? Where should we go? Nat. Immunol. 2001, 2, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Kitz, A.; Singer, E.; Hafler, D. Regulatory T Cells: From Discovery to Autoimmunity. Cold Spring Harb. Perspect. Med. 2018, 8, a029041. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.; Findling, O. An overview of pivotal trials and real-world evidence for CD20-depleting therapy in multiple sclerosis. Wien. Med. Wochenschr. 2022, 172, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, J.T.; Tsirka, S.E. Advances in immunotherapeutic research for glioma therapy. J. Neurol. 2018, 265, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Kordower, J.H. Immunotherapy in Parkinson’s disease: Current status and future directions. Neurobiol. Dis. 2019, 132, 104587. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, T.; Huang, Q.; Jin, W.; Chen, J. Immunotherapy for Malignant Glioma: Current Status and Future Directions. Trends Pharmacol. Sci. 2020, 41, 123–138. [Google Scholar] [CrossRef]
- Kozłowski, M.; Borzyszkowska, D.; Cymbaluk-Płoska, A. The Role of TIM-3 and LAG-3 in the Microenvironment and Immunotherapy of Ovarian Cancer. Biomedicines 2022, 10, 2826. [Google Scholar] [CrossRef]
- Kuppuswamy, P.S.; Takala, C.R.; Sola, C.L. Management of psychiatric symptoms in anti-NMDAR encephalitis: A case series, literature review and future directions. Gen. General. Hosp. Psychiatry 2014, 36, 388–391. [Google Scholar] [CrossRef]
- Shi, J.; Hua, L.; Harmer, D.; Li, P.; Ren, G. Cre Driver Mice Targeting Macrophages. Methods Mol. Biol. 2018, 1784, 263–275. [Google Scholar] [CrossRef]
- McAllister, A.K.; Patterson, P.H. Neuroimmunology in Brain Development and Disease. Dev. Neurobiol. 2012, 72, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Park, M.J.; Kwon, M.S. Central Nervous System-Peripheral Immune System Dialogue in Neurological Disorders: Possible Application of Neuroimmunology in Urology. Int. Neurourol. J. 2016, 20 (Suppl. S1), S8–S14. [Google Scholar] [CrossRef]
- Norden, D.M.; Muccigrosso, M.M.; Godbout, J.P. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 2015, 96, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Archer, T.; Fredriksson, A.; Schϋtz, E.; Kostrzewa, R.M. Influence of Physical Exercise on Neuroimmunological Functioning and Health: Aging and Stress. Neurotox. Res. 2011, 20, 69–83. [Google Scholar] [CrossRef]
- Fonken, L.K.; Gaudet, A.D. Neuroimmunology of healthy brain aging. Curr. Opin. Neurobiol. 2022, 77, 102649. [Google Scholar] [CrossRef] [PubMed]
- Lucin, K.M.; Wyss-Coray, T. Immune activation in brain aging and neurodegeneration: Too much or too little? Neuron 2009, 64, 110–122. [Google Scholar] [CrossRef]
- Tenembaum, S.N. Pediatric demyelinating disease and anti-MOG antibody. Clin. Exp. Neuroimmunol. 2021, 12, 7–21. [Google Scholar] [CrossRef]
- Nosadini, M.; Alper, G.; Riney, C.J.; Benson, L.A.; Mohammad, S.S.; Ramanathan, S.; Nolan, M.; Appleton, R.; Leventer, R.J.; Deiva, K.; et al. Rituximab monitoring and redosing in pediatric neuromyelitis optica spectrum disorder. Neurol.-Neuroimmunol. Neuroinflamm. 2016, 3, e188. [Google Scholar] [CrossRef]
- Reinert, M.-C.; Benkert, P.; Wuerfel, J.; Michalak, Z.; Ruberte, E.; Barro, C.; Huppke, P.; Stark, W.; Kropshofer, H.; Tomic, D.; et al. Serum neurofilament light chain is a useful biomarker in pediatric multiple sclerosis. Neurol.-Neuroimmunol. Neuroinflamm. 2020, 7, e749. [Google Scholar] [CrossRef]
- Sguigna, P.V.; McCreary, M.C.; Conger, D.L.; Graves, J.S.; Benson, L.A.; Waldman, A.T.; Greenberg, B.M.; on behalf of the PERCEPTION Collaboration. Utilization of Visual Acuity Retroilluminated Charts for the Assessment of Afferent Visual System Dysfunction in a Pediatric Neuroimmunology Population. J. Neuro-Ophthalmol. 2021, 41, 19. [Google Scholar] [CrossRef]
- Polyakova, V.O.; Kvetnoy, I.M.; Anderson, G.; Rosati, J.; Mazzoccoli, G.; Linkova, N.S. Reciprocal Interactions of Mitochondria and the Neuroimmunoendocrine System in Neurodegenerative Disorders: An Important Role for Melatonin Regulation. Front. Physiol. 2018, 9, 199. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2018.00199 (accessed on 25 July 2023). [CrossRef] [PubMed]
- Akmaev, I.G. Current concepts of the interactions of regulating systems: Nervous, endocrine and immune. Usp. Fiziol. Nauk. 1996, 27, 3–20. [Google Scholar]
- Kvetnoy, I.M. Neuroimmunoendocrinology: Where is the field for study? Neuroendocrinol. Lett. 2002, 23, 119–120. [Google Scholar]
- Wilder, R.L. Neuroimmunoendocrinology of the Rheumatic Diseases. Ann. N. Y. Acad. Sci. 2002, 966, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Aguilar, M.; Rondón, R. Neuroimmunoendocrine System in Health and Disease. EC Microbiol. 2018, 14, 2–6. [Google Scholar]
- Anderson, G.; Maes, M. Neurodegeneration in Parkinson’s Disease: Interactions of Oxidative Stress, Tryptophan Catabolites and Depression with Mitochondria and Sirtuins. Mol. Neurobiol. 2014, 49, 771–783. [Google Scholar] [CrossRef]
- Akmayev, I.G. Neuroimmunoendocrinology: Evidence and hypothesis. Probl. Endocrinol. 1997, 43, 3–9. [Google Scholar] [CrossRef]
- Nguyen, M.; Palm, N.W. Gut instincts in neuroimmunity from the eighteenth to twenty-first centuries. Semin. Immunopathol. 2022, 44, 569–579. [Google Scholar] [CrossRef]
- Cui, C.; Ruan, Y.; Qiu, W. Potential role of the gut microbiota in neuromyelitis optica spectrum disorder: Implication for intervention. J. Clin. Neurosci. 2020, 82, 193–199. [Google Scholar] [CrossRef]
- van Thiel, I.A.M.; de Jonge, W.J.; Chiu, I.M.; van den Wijngaard, R.M. Microbiota-neuroimmune cross talk in stress-induced visceral hypersensitivity of the bowel. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G1034–G1041. [Google Scholar] [CrossRef]
- Maranduba, C.M.d.C.; De Castro, S.B.R.; de Souza, G.T.; Rossato, C.; da Guia, F.C.; Valente, M.A.S.; Rettore, J.V.P.; Maranduba, C.P.; de Souza, C.M.; Carmo, A.M.R.D.; et al. Intestinal Microbiota as Modulators of the Immune System and Neuroimmune System: Impact on the Host Health and Homeostasis. J. Immunol. Res. 2015, 2015, e931574. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eva, L.; Pleș, H.; Covache-Busuioc, R.-A.; Glavan, L.A.; Bratu, B.-G.; Bordeianu, A.; Dumitrascu, D.-I.; Corlatescu, A.D.; Ciurea, A.V. A Comprehensive Review on Neuroimmunology: Insights from Multiple Sclerosis to Future Therapeutic Developments. Biomedicines 2023, 11, 2489. https://doi.org/10.3390/biomedicines11092489
Eva L, Pleș H, Covache-Busuioc R-A, Glavan LA, Bratu B-G, Bordeianu A, Dumitrascu D-I, Corlatescu AD, Ciurea AV. A Comprehensive Review on Neuroimmunology: Insights from Multiple Sclerosis to Future Therapeutic Developments. Biomedicines. 2023; 11(9):2489. https://doi.org/10.3390/biomedicines11092489
Chicago/Turabian StyleEva, Lucian, Horia Pleș, Razvan-Adrian Covache-Busuioc, Luca Andrei Glavan, Bogdan-Gabriel Bratu, Andrei Bordeianu, David-Ioan Dumitrascu, Antonio Daniel Corlatescu, and Alexandru Vlad Ciurea. 2023. "A Comprehensive Review on Neuroimmunology: Insights from Multiple Sclerosis to Future Therapeutic Developments" Biomedicines 11, no. 9: 2489. https://doi.org/10.3390/biomedicines11092489
APA StyleEva, L., Pleș, H., Covache-Busuioc, R. -A., Glavan, L. A., Bratu, B. -G., Bordeianu, A., Dumitrascu, D. -I., Corlatescu, A. D., & Ciurea, A. V. (2023). A Comprehensive Review on Neuroimmunology: Insights from Multiple Sclerosis to Future Therapeutic Developments. Biomedicines, 11(9), 2489. https://doi.org/10.3390/biomedicines11092489