The Effects of Adiponectin on the Behavior of B-Cell Leukemia Cells: Insights from an In Vitro Study
Abstract
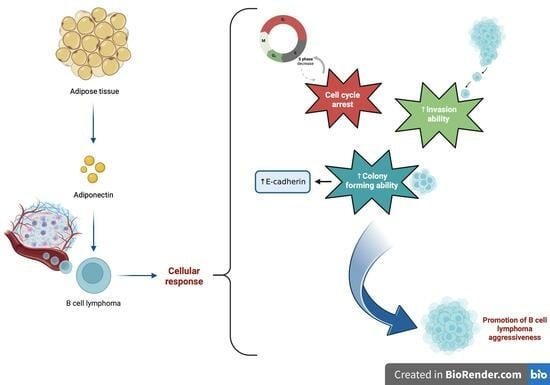
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assays
2.3. Cell Cycle Analysis
2.4. RNA Extraction and Quantitative Real Time-PCR
2.5. Western Blot Assay
2.6. Colony Formation Assay
2.7. Matrigel Matrix Invasion Assay
2.8. Statistical Analysis
3. Results
3.1. Adiponectin Receptors Are Expressed in JVM-2 Cells with Up-Regulation at the mRNA and Protein Levels of AdipoR1 Induced via the Administration of AdipoRon
3.2. AdipoRon Reduces JVM-2 Cell Viability, Inducing a Cytostatic Effect without Affecting Apoptotic and Autophagy Pathways
3.3. AdipoRon Exposure Promotes Colony Formation and Induces E-Cadherin Protein Expression on JVM-2 Cells
3.4. AdipoRon Increases the Invasive Ability of JVM-2 Cells Compared to Untreated Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar] [CrossRef]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef]
- Brasil, S.A.B.; Colaço, C.; Barrese, T.; Paes, R.P.; Bortolheiro, C.; Cançado, R.D. Non indolent mantle cell lymphoma at a single public hospital in Brazil: Realworld first-line treatment cohort study data. Hematol. Transfus. Cell Ther. 2020, 42, 93–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Zheng, T.; Ma, S. Risk Factors of Non-Hodgkin Lymphoma. Expert Opin. Med. Diagn. 2011, 5, 539–550. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Nigro, E.; Mallardo, M.; Polito, R.; Scialò, F.; Bianco, A.; Daniele, A. Adiponectin and Leptin Exert Antagonizing Effects on HUVEC Tube Formation and Migration Modulating the Expression of CXCL1, VEGF, MMP-2 and MMP-9. Int. J. Mol. Sci. 2021, 22, 7516. [Google Scholar] [CrossRef]
- Liu, M.; Liu, F. Regulation of adiponectin multimerization, signaling and function. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 25–31. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, M. Adiponectin: A versatile player of innate immunity. J. Mol. Cell Biol. 2016, 8, 120–128. [Google Scholar] [CrossRef]
- Yokota, T.; Meka, C.S.R.; Kouro, T.; Medina, K.L.; Igarashi, H.; Takahashi, M.; Oritani, K.; Funahashi, T.; Tomiyama, Y.; Matsuzawa, Y.; et al. Adiponectin, a Fat Cell Product, Influences the Earliest Lymphocyte Precursors in Bone Marrow Cultures by Activation of the Cyclooxygenase-Prostaglandin Pathway in Stromal Cells. J. Immunol. 2003, 171, 5091–5099. [Google Scholar] [CrossRef]
- Lago, F.; Dieguez, C.; Gómez-Reino, J.; Gualillo, O. Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pract. Rheumatol. 2007, 3, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.T.; Dessypris, N.; Panagopoulou, P.; Sergentanis, T.N.; Mentis, A.-F.A.; Pourtsidis, A.; Polychronopoulou, S.; Kalmanti, M.; Athanasiadou-Piperopoulou, F.; Moschovi, M. Adipocytokines in relation to Hodgkin lymphoma in children. Pediatr. Blood Cancer 2009, 54, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Pamuk, G.E.; Turgut, B.; Demir, M.; Vural, O. Increased adiponectin level in non-Hodgkin lymphoma and its relationship with interleukin-10. Correlation with clinical features and outcome. J. Exp. Clin. Cancer Res. 2006, 25, 537–541. [Google Scholar] [PubMed]
- Conroy, S.M.; Maskarinec, G.; Morimoto, Y.; Franke, A.A.; Cooney, R.V.; Wilkens, L.R.; Goodman, M.T.; Hernadez, B.Y.; Le Marchand, L.; Henderson, B.E.; et al. Non-Hodgkin Lymphoma and Circulating Markers of Inflammation and Adiposity in a Nested Case–Control Study: The Multiethnic Cohort. Cancer Epidemiol. Biomark. Prev. 2013, 22, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Obeid, S.; Hebbard, L. Role of Adiponectin and Its Receptors in Cancer. Cancer Biol. Med. 2012, 9, 213–220. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Polito, R.; Bartollino, S.; Nigro, E.; Porcile, C.; Bianco, A.; Daniele, A.; Moncharmont, B. Adiponectin as Link Factor between Adipose Tissue and Cancer. Int. J. Mol. Sci. 2019, 20, 839. [Google Scholar] [CrossRef]
- Mallardo, M.; Signoriello, E.; Lus, G.; Daniele, A.; Nigro, E. Adiponectin Alleviates Cell Injury due to Cerebrospinal Fluid from Multiple Sclerosis Patients by Inhibiting Oxidative Stress and Proinflammatory Response. Biomedicines 2023, 11, 1692. [Google Scholar] [CrossRef]
- Akimoto, M.; Maruyama, R.; Kawabata, Y.; Tajima, Y.; Takenaga, K. Antidiabetic adiponectin receptor agonist AdipoRon suppresses tumour growth of pancreatic cancer by inducing RIPK1/ERK-dependent necroptosis. Cell Death Dis. 2018, 9, 804. [Google Scholar] [CrossRef]
- Nigro, E.; Pecoraro, M.T.; Formato, M.; Piccolella, S.; Ragucci, S.; Mallardo, M.; Russo, R.; Di Maro, A.; Daniele, A.; Pacifico, S. Cannabidiolic acid in Hemp Seed Oil Table Spoon and Beyond. Molecules 2022, 27, 2566. [Google Scholar] [CrossRef]
- Nigro, E.; Orlandella, F.M.; Polito, R.; Mariniello, R.M.; Monaco, M.L.; Mallardo, M.; De Stefano, A.E.; Iervolino, P.L.C.; Salvatore, G.; Daniele, A. Adiponectin and leptin exert antagonizing effects on proliferation and motility of papillary thyroid cancer cell lines. J. Physiol. Biochem. 2021, 77, 237–248. [Google Scholar] [CrossRef]
- Tsartsalis, A.N.; Tagka, A.; Kotoulas, A.; Mirkopoulou, D.; Geronikolou, S.A.G.; Lambrou, G. Adiponectin and Its Effects on Acute Leukemia Cells: An Experimental and Bioinformatics Approach. Adv. Exp. Med. Biol. 2021, 1338, 117–127. [Google Scholar] [CrossRef]
- Dalamaga, M.; Christodoulatos, G.S. Adiponectin as a biomarker linking obesity and adiposopathy to hematologic malignancies. Horm. Mol. Biol. Clin. Investig. 2015, 23, 5–20. [Google Scholar] [CrossRef]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The Role of Adiponectin in Cancer: A Review of Current Evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef]
- Clarke, P.R.; Allan, L.A. Cell-cycle control in the face of damage—A matter of life or death. Trends Cell Biol. 2009, 19, 89–98. [Google Scholar] [CrossRef]
- Pham, D.V.; Raut, P.K.; Pandit, M.; Chang, J.H.; Katila, N.; Choi, D.Y.; Jeong, J.H.; Park, P.H. Globular Adiponectin Inhibits Breast Cancer Cell Growth through Modulation of Inflammasome Activation: Critical Role of Sestrin2 and AMPK Signaling. Cancers 2020, 12, 613. [Google Scholar] [CrossRef]
- Taliaferro-Smith, L.; Nagalingam, A.; Knight, B.B.; Oberlick, E.; Saxena, N.K.; Sharma, D. Integral Role of PTP1B in Adiponectin-Mediated Inhibition of Oncogenic Actions of Leptin in Breast Carcinogenesis. Neoplasia 2013, 15, 23-IN11. [Google Scholar] [CrossRef]
- Moon, H.S.; Mantzoros, C.S. Adiponectin and metformin additively attenuate IL1β-induced malignant potential of colon cancer. Endocr. Relat. Cancer 2013, 20, 849–859. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Lewis-Tuffin, L.J.; Anastasiadis, P.Z. E-cadherin’s dark side: Possible role in tumor progression. Biochim. Biophys. Acta 2012, 1826, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.X.; An, X.Z.; Li, P.F.; Li, Y.J.; Cui, Y.H.; Tang, X.; Liu, Q.H.; Hu, Y.N.; Liu, W.; Liang, S.Y.; et al. E-cadherin Expression in Children with Acute Leukemia and Its Clinical Significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019, 27, 339–347. [Google Scholar] [PubMed]
- Qi, F.Q.; Han, W.; Yan, J.; Xin, C.; Li, Y.; Guo, L.; Wang, W.P.; Gao, J.Z. Influence of E-cadherin methylation on prognosis in children with acute lymphoblastic leukemia. Zhongguo Dang Dai Er Ke Za Zhi = Chin. J. Contemp. Pediatr. 2023, 25, 46–50. [Google Scholar]
- Dong, L.L.; Liu, L.; Ma, C.H.; Li, J.S.; Du, C.; Xu, S.; Han, L.H.; Li, L.; Wang, X.W. E-cadherin promotes proliferation of human ovarian cancer cells in vitro via activating MEK/ERK pathway. Acta Pharmacol. Sin. 2012, 33, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Naimo, G.D.; Forestiero, M.; Paolì, A.; Malivindi, R.; Gelsomino, L.; Győrffy, B.; Leonetti, A.E.; Giordano, F.; Panza, S.; Conforti, F.L.; et al. ERα/LKB1 complex upregulates E-cadherin expression and stimulates breast cancer growth and progression upon adiponectin exposure. Int. J. Cancer 2023, 153, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Kantak, S.S.; Kramer, R.H. E-cadherin Regulates Anchorage-independent Growth and Survival in Oral Squamous Cell Carcinoma Cells. J. Biol. Chem. 1998, 273, 16953–16961. [Google Scholar] [CrossRef] [PubMed]
- Izadi, V.; Farabad, E.; Azadbakht, L. Serum adiponectin level and different kinds of cancer: A review of recent evidence. J. Family Med. Prim. Care. 2018, 7, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Sapio, L.; Nigro, E.; Ragone, A.; Salzillo, A.; Illiano, M.; Spina, A.; Polito, R.; Daniele, A.; Naviglio, S. AdipoRon Affects Cell Cycle Progression and Inhibits Proliferation in Human Osteosarcoma Cells. J. Oncol. 2020, 2020, 7262479. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Sarnataro, D.; Mazzarella, G.; Sofia, M.; Bianco, A.; Daniele, A. Adiponectin affects lung epithelial A549 cell viability counteracting TNFα and IL-1ß toxicity through AdipoR1. Int. J. Biochem. Cell Biol. 2013, 45, 1145–1153. [Google Scholar] [CrossRef]
- Ramzan, A.A.; Bitler, B.G.; Hicks, D.; Barner, K.; Qamar, L.; Behbakht, K.; Powell, T.; Jansson, T.; Wilson, H. Adiponectin receptor agonist AdipoRon induces apoptotic cell death and suppresses proliferation in human ovarian cancer cells. Mol. Cell. Biochem. 2019, 461, 37–46. [Google Scholar] [CrossRef]
- Li, Y.; Jin, R.; Li, L.; Hsu, H.H.; You, I.C.; Yoon, H.J.; Yoon, K.C. Therapeutic Effect of Topical Adiponectin-Derived Short Peptides Compared with Globular Adiponectin in Experimental Dry Eye and Alkali Burn. J. Ocul. Pharmacol. Ther. 2020, 36, 88–96. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallardo, M.; Scalia, G.; Raia, M.; Daniele, A.; Nigro, E. The Effects of Adiponectin on the Behavior of B-Cell Leukemia Cells: Insights from an In Vitro Study. Biomedicines 2023, 11, 2585. https://doi.org/10.3390/biomedicines11092585
Mallardo M, Scalia G, Raia M, Daniele A, Nigro E. The Effects of Adiponectin on the Behavior of B-Cell Leukemia Cells: Insights from an In Vitro Study. Biomedicines. 2023; 11(9):2585. https://doi.org/10.3390/biomedicines11092585
Chicago/Turabian StyleMallardo, Marta, Giulia Scalia, Maddalena Raia, Aurora Daniele, and Ersilia Nigro. 2023. "The Effects of Adiponectin on the Behavior of B-Cell Leukemia Cells: Insights from an In Vitro Study" Biomedicines 11, no. 9: 2585. https://doi.org/10.3390/biomedicines11092585
APA StyleMallardo, M., Scalia, G., Raia, M., Daniele, A., & Nigro, E. (2023). The Effects of Adiponectin on the Behavior of B-Cell Leukemia Cells: Insights from an In Vitro Study. Biomedicines, 11(9), 2585. https://doi.org/10.3390/biomedicines11092585