Sulfated CXCR3 Peptide Trap Use as a Promising Therapeutic Approach for Age-Related Macular Degeneration
Abstract
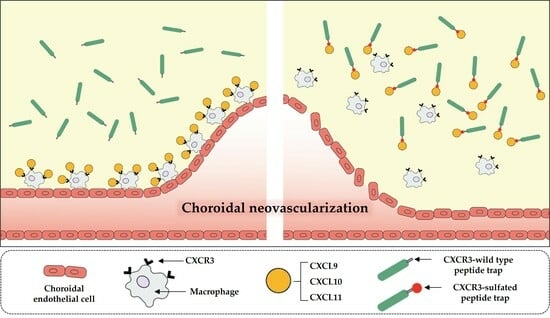
:1. Introduction
2. Materials and Methods
2.1. Plasmid Construction
2.2. Protein Purification
2.3. Wound Healing Assay
2.4. Invasion Assay
2.5. RAW 264.7 Cell Culture
2.6. Experimental Animals
2.7. Laser-Induced CNV Mouse Models
2.8. Quantitative Reverse Transcriptase-Mediated Real-Time PCR (qPCR)
2.9. Quantitation of CNV in the Mouse Model of Laser-Induced CNV
2.10. Immunofluorescence Staining
2.11. Statistical Analysis
3. Results
3.1. Cloning and Purification of mCXCR3-S2 and hCXCR3-S2
3.2. hCXCR3 Sulfation Attenuated CXCL10-Induced Cell Migration and Invasion

3.3. mCXCR3-S2 Prevented CNV and Macrophage Infiltration in the Laser-Induced CNV Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambati, J.; Anand, A.; Fernandez, S.; Sakurai, E.; Lynn, B.C.; Kuziel, W.A.; Rollins, B.J.; Ambati, B.K. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med. 2003, 11, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Atkinson, J.; Gelfand, G. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Liew, G.; Mitchell, P. Clinical update: New treatments for age-related macular degeneration. Lancet 2007, 9583, s0140–s6736. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.S.; Schwartz, S.D.; Hubschman, J.P. Age-related macular degeneration: Current and novel therapies. Maturitas 2010, 1, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, I.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 2, 134–143. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 2, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Webers, A.; Heneka, M.T.; Gleeson, P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020, 1, 28–41. [Google Scholar] [CrossRef]
- Anand, A.; Sharma, N.K.; Gupta, A.; Prabhakar, S.; Sharma, S.K.; Singh, R.; Gupta, P.K. Single nucleotide polymorphisms in MCP-1 and its receptor are associated with the risk of age related macular degeneration. PLoS ONE 2012, 7, e49905. [Google Scholar] [CrossRef]
- Vandercappellen, J.; Van Damme, J.; Struyf, S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008, 2, 226–244. [Google Scholar] [CrossRef]
- Satarkar, D.; Patra, C. Evolution, Expression and Functional Analysis of CXCR3 in Neuronal and Cardiovascular Diseases: A Narrative Review. Front. Cell Dev. Biol. 2022, 20, 882017. [Google Scholar] [CrossRef]
- Loetscher, M.; Gerber, B.; Loetscher, P.; Jones, S.A.; Piali, L.; Clark, L.I. Chemokine Receptor Specific for IP10 and Mig: Structure, Function, and Expression in Activated T-Lymphocytes. J. Exp. Med. 1996, 184, 963–969. [Google Scholar] [CrossRef]
- Lasagni, L.; Francalanci, M.; Annunziato, F.; Lazzeri, E.; Giannini, S.; Cosmi, L. An Alternatively Spliced Variant of CXCR3 Mediates the Inhibition of Endothelial Cell Growth Induced by IP-10, Mig, and I-TAC, and Acts as Functional Receptor for Platelet Factor 4. J. Exp. Med. 2003, 197, 1537–1549. [Google Scholar] [CrossRef]
- Colvin, R.A.; Campanella, G.S.V.; Manice, L.A.; Luster, A.D. CXCR3 Requires Tyrosine Sulfation for Ligand Binding and a Second Extracellular Loop Arginine Residue for Ligand-Induced Chemotaxis. Mol. Cell. Biol. 2006, 26, 5838–5849. [Google Scholar] [CrossRef]
- Mo, F.M.; Proia, A.D.; Johnson, W.H.; Cyr, D.; Lashkari, K. Interferon gamma-inducible protein-10 (IP-10) and eotaxin as biomarkers in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 8, 4226–4236. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, Y.; Nakamura, Y.; Yoneyama, S.; Mabuchi, F.; Gotoh, T.; Tateno, Y.; Sugiyama, A.; Kubota, T.; Iijima, H. Aqueous humor cytokine levels in patients with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Ophthalmic Res. 2015, 1, 2–7. [Google Scholar] [CrossRef]
- Fujimura, S.; Takahashi, H.; Yuda, K.; Ueta, T.; Iriyama, A.; Inoue, T.; Kaburaki, T.; Tamaki, Y.; Matsushima, K.; Yanagi, Y. Angiostatic effect of CXCR3 expressed on choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1999–2006. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor. Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef]
- Veldkamp, C.T.; Seibert, C.; Peterson, F.C.; De la Cruz, N.B.; Haugner, J.C.; Basnet, H.; Sakmar, T.P.; Volkman, B.F. Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci. Signal. 2008, 37, ra4. [Google Scholar] [CrossRef]
- Zhu, J.Z.; Millard, C.J.; Ludeman, J.P.; Simpson, L.S.; Clayton, D.J.; Payne, R.J.; Widlanski, T.S.; Stone, M.J. Tyrosine sulfation influences the chemokine binding selectivity of peptides derived from chemokine receptor CCR3. Biochemistry 2011, 9, 1524–1534. [Google Scholar] [CrossRef]
- Stemmer, W.P.; Crameri, A.; Ha, K.D.; Brennan, T.M.; Heyneker, H.L. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene 1995, 164, 49–53. [Google Scholar] [CrossRef]
- Liu, C.C.; Cellitti, S.E.; Geierstanger, B.H.; Schultz, P.G. Efficient expression of tyrosine-sulfated proteins in E. coli using an expanded genetic code. Nat. Protoc. 2009, 12, 1784–1789. [Google Scholar] [CrossRef]
- Choi, J.; Ahn, S.S.; Lim, Y.; Lee, Y.H.; Shin, S.Y. Inhibitory effect of Alisma canaliculatum ethanolic extract on NF-Κb-dependent CXCR3 and CXCL10 expression in TNFα-exposed MDA-MB-231 breast cancer cells. Int. J. Mol. Sci. 2018, 9, 2607. [Google Scholar] [CrossRef]
- Wu, Z.; Han, X.; Yan, J.; Pan, Y.; Gong, J.; Di, J.; Cheng, Z.; Jin, Z.; Wang, Z.; Zheng, Q.; et al. The prognostic significance of chemokine receptor CXCR3 expression in colorectal carcinoma. Biomed. Pharmacother. 2012, 5, 373–377. [Google Scholar] [CrossRef]
- Windmüller, C.; Zech, D.; Avril, S.; Boxberg, M.; Dawidek, T.; Schmalfeldt, B.; Schmitt, M.; Kiechle, M.; Bronger, H. CXCR3 mediates ascites-directed tumor cell migration and predicts poor outcome in ovarian cancer patients. Oncogenesis 2017, 5, e331. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, S.; Ni, H.; Zhao, P.; Chen, G.; Xu, B.; Yuan, L. The role of CXCR3 and its ligands in cancer. Front. Oncol. 2022, 12, 1022688. [Google Scholar] [CrossRef]
- Vincenzo, T.; Chao, C.; Ralf, B.; Jan, P.B.; Astrid, M.S.; Martine, J.S.; Marco, S.; Herman, P.S.; Annemarie, H.M. The CXCR3-CXCL10 signaling axis mediates macrophage recruitment and dis semination of mycobacterial infection. Dis. Model. Mech. 2015, 8, 253–269. [Google Scholar]
- Xin, J.L.; Qiang, C.; Ye, J.R.; Feng, C.; Jiong, C. CXCR3.1 and CXCR3.2 Differentially Contribute to Macrophage Polarization in Teleost Fish. J. Immunol. 2017, 198, 4692–4706. [Google Scholar] [CrossRef]
- Tatsuya, H.; Venkata, S.V.; Yoshio, Y.; Masato, N.; Shigeto, S.; Shigeki, S.; Satoru, Y.; Hideki, K.; Itaru, M.; Yuichiro, N.; et al. Inhibition of the CXCL9-CXCR3 axis suppresses the progression of experimental apical periodontitis by blocking macrophage migration and activation. Sci. Rep. 2021, 11, 2613. [Google Scholar] [CrossRef]
- Monteagudo, C.; Martin, J.M.; Jorda, E.; Llombart-Bosch, A. CXCR3 chemokinereceptor immunoreactivity in primary cutaneous malignant melanoma: Correlationwith clinicopathological prognostic factors. J. Clin. Pathol. 2007, 60, 596–599. [Google Scholar] [CrossRef]
- Kawada, K.; Hosogi, H.; Sonoshita, M.; Sakashita, H.; Manabe, T.; Shimahara, Y.; Sakai, Y.; Takabayashi, A.; Oshima, M.; Taketo, M.M. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymphnodes. Oncogene 2007, 26, 4679–4688. [Google Scholar] [CrossRef]
- Ma, X.; Norsworthy, K.; Kundu, N.; Rodgers, W.H.; Gimotty, P.A.; Goloubeva, O.; Lipsky, M.; Li, Y.; Holt, D.; Fulton, A. CXCR3 expression is associated with poor survival in breast cancer andpromotes metastasis in a murine model. Mol. Cancer Ther. 2009, 8, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, C.T.; Seibert, C.; Peterson, F.C.; Sakmar, T.P.; Volkman, B.F. Recognition of a CXCR4 sulfotyrosine by the chemokine stromal cell-derived factor-1(SDF-1/CXCL12). J. Mol. Biol. 2006, 359, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Duma, L.; Häussinger, D.; Rogowski, M.; Lusso, P.; Grzesiek, S. Recognition of RANTES by extracellular parts of the CCR5 receptor. J. Mol. Biol. 2007, 365, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.S.; Zhu, J.Z.; Widlanski, T.S.; Stone, M.J. Regulation of chemokine recognition by site-specific tyrosine sulfation of receptor peptides. Chem. Biol. 2009, 16, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Seibert, C.; Veldkamp, C.T.; Peterson, F.C.; Chait, B.T.; Volkman, B.F.; Sakmar, T.P. Sequential tyrosine sulfation of CXCR4 by tyrosylprotein sulfotransferases. Biochemistry 2008, 47, 11251–11262. [Google Scholar] [CrossRef]
- Gao, J.M.; Xiang, R.L.; Jiang, L.; Li, W.H.; Feng, Q.P.; Guo, Z.J.; Sun, Q.; Zeng, Z.P.; Fang, F.D. Sulfated tyrosines 27 and 29 in the N-terminus of human CXCR3 participate in binding native IP-10. Acta Pharmacol. Sin. 2009, 30, 193–201. [Google Scholar] [CrossRef]
- Denoyer, A.; Godefroy, D.; Celerier, I.; Frugier, J.; Degardin, J.; Harrison, J.K.; Brignole-Baudouin, F.; Picaud, S.; Baleux, F.; Sahel, J.A.; et al. CXCR3 antagonism of SDF-1(5-67) restores trabecular function and prevents retinal neurodegeneration in a rat model of ocular hypertension. PLoS ONE 2012, 7, e37873. [Google Scholar] [CrossRef]
- Ludeman, J.P.; Stone, M.J. The structural role of receptor tyrosine sulfation in chemokine recognition. Br. J. Pharmacol. 2014, 5, 1167–1179. [Google Scholar] [CrossRef]
- Millard, C.J.; Ludeman, J.P.; Canals, M.; Bridgford, J.L.; Hinds, M.G.; Clayton, D.J.; Christopoulos, A.; Payne, R.J.; Stone, M.J. Structural basis of receptor sulfotyrosine recognition by a CC chemokine: The N-terminal region of CCR3 bound to CCL11/eotaxin-1. Structure 2014, 11, 1571–1581. [Google Scholar] [CrossRef]
- Espinosa-Heidmann, D.G.; Suner, I.J.; Hernandez, E.P.; Monroy, D.; Csaky, K.G.; Cousins, S.W. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2003, 8, 3586–3592. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, F.; Tang, M.; Yuan, M.; Hu, A.; Zhan, Z.; Li, Z.; Li, J.; Ding, X.; Lu, L. Macrophage polarization in experimental and clinical choroidal neovascularization. Sci. Rep. 2016, 6, 30933. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Takagi, H.; Takagi, C.; Suzuma, K.; Otani, A.; Ishida, K.; Matsumura, M.; Ogura, Y.; Honda, Y. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1999, 9, 1891–1898. [Google Scholar]
- Yang, X.; Zhao, L.; Campos, M.; Abu-Asab, M.; Ortolan, D.; Hotaling, N.; Bharti, K.; Wong, W.T. Choroid-resident macrophages maintain local vasculature and RPE integrity and spontaneously regenerate following depletion. bioRxiv 2019. [Google Scholar] [CrossRef]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2015, 1, 61–69. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, G.; Chae, J.-B.; Jung, S.-A.; Lyu, J.; Chung, H.; Lee, J.H. Sulfated CXCR3 Peptide Trap Use as a Promising Therapeutic Approach for Age-Related Macular Degeneration. Biomedicines 2024, 12, 241. https://doi.org/10.3390/biomedicines12010241
Jo G, Chae J-B, Jung S-A, Lyu J, Chung H, Lee JH. Sulfated CXCR3 Peptide Trap Use as a Promising Therapeutic Approach for Age-Related Macular Degeneration. Biomedicines. 2024; 12(1):241. https://doi.org/10.3390/biomedicines12010241
Chicago/Turabian StyleJo, Gukheui, Jae-Byoung Chae, Sun-Ah Jung, Jungmook Lyu, Hyewon Chung, and Joon H. Lee. 2024. "Sulfated CXCR3 Peptide Trap Use as a Promising Therapeutic Approach for Age-Related Macular Degeneration" Biomedicines 12, no. 1: 241. https://doi.org/10.3390/biomedicines12010241
APA StyleJo, G., Chae, J. -B., Jung, S. -A., Lyu, J., Chung, H., & Lee, J. H. (2024). Sulfated CXCR3 Peptide Trap Use as a Promising Therapeutic Approach for Age-Related Macular Degeneration. Biomedicines, 12(1), 241. https://doi.org/10.3390/biomedicines12010241