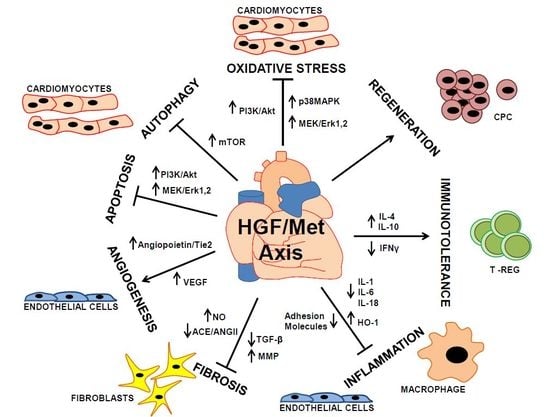
HGF/Met Axis in Heart Function and Cardioprotection
Abstract
:1. Introduction
2. The Role of Hepatocyte Growth Factor (HGF)/Met in Physiological Heart Development
3. The Potential Cardiotoxicity of HGF/Met Inhibitors
4. The Cardioprotective Role of HGF/Met in Myocardial Infarction
5. Anti-Apoptotic and Anti-Autophagic Function of HGF in Cardiomyocytes
6. Proangiogenic Function of HGF toward Vascular Cells
7. Anti-Fibrotic Action of HGF in Cardiac Fibroblasts
8. Anti-Inflammatory and Immunomodulatory Function of HGF
9. HGF and Cardiac Regeneration
10. The Putative Role of Elevated HGF as Prognostic Marker of Severity in Patients with Cardiac Diseases
11. Conclusions and Future Perspectives

Acknowledgments
Conflicts of Interest
References
- Rappolee, D.A.; Iyer, A.; Patel, Y. Hepatocyte growth factor and its receptor are expressed in cardiac myocytes during early cardiogenesis. Circ. Res. 1996, 78, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Song, W.M.; Majka, S.M.; McGuire, P.G. Hepatocyte growth factor expression in the developing myocardium: Evidence for a role in the regulation of the mesenchymal cell phenotype and urokinase expression. Dev. Dyn. 1999, 214, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Bladt, F.; Riethmacher, D.; Isenmann, S.; Aguzzi, A.; Birchmeier, C. Essential role for the c-Met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995, 376, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Bladt, F.; Goedecke, S.; Brinkmann, V.; Zschiesche, W.; Sharpe, M.; Gherardi, E.; Birchmeier, C. Scatter factor/hepatocyte growth-factor is essential for liver development. Nature 1995, 373, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Minowa, O.; Mori, C.; Shiota, K.; Kuno, J.; Noda, T.; Kitamura, N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995, 373, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Arechederra, M.; Carmona, R.; Gonzalez-Nunez, M.; Gutierrez-Uzquiza, A.; Bragado, P.; Cruz-Gonzalez, I.; Cano, E.; Guerrero, C.; Sanchez, A.; Lopez-Novoa, J.M.; et al. Met signaling in cardiomyocytes is required for normal cardiac function in adult mice. Biochim. Biophys. Acta 2013, 1832, 2204–2215. [Google Scholar] [CrossRef]
- Leo, C.; Sala, V.; Morello, M.; Chiribiri, A.; Riess, I.; Mancardi, D.; Schiaffino, S.; Ponzetto, C.; Crepaldi, T. Activated Met signalling in the developing mouse heart leads to cardiac disease. PLoS One 2011, 6, e14675. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Leo, C.; Gallo, S.; Sala, V.; Bucci, E.; Natale, M.; Cantarella, D.; Medico, E.; Crepaldi, T. Gene expression profiling of HGF/Met activation in neonatal mouse heart. Transgenic Res. 2013, 22, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Sala, V.; Gallo, S.; Leo, C.; Gatti, S.; Gelb, B.D.; Crepaldi, T. Signaling to cardiac hypertrophy: Insights from human and mouse RASopathies. Mol. Med. 2012, 18, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Comoglio, P.M.; Giordano, S.; Trusolino, L. Drug development of MET inhibitors: Targeting oncogene addiction and expedience. Nat. Rev. Drug Discover. 2008, 7, 504–516. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Force, T.; Ewer, M.S.; de Keulenaer, G.W.; Suter, T.M.; Anker, S.D.; Avkiran, M.; de Azambuja, E.; Balligand, J.L.; Brutsaert, D.L.; et al. Cardiovascular side effects of cancer therapies: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2011, 13, 1–10. [Google Scholar] [CrossRef]
- Cecchi, F.; Rabe, D.C.; Bottaro, D.P. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin. Ther. Tar. 2012, 16, 553–572. [Google Scholar] [CrossRef]
- Doherty, K.R.; Wappel, R.L.; Talbert, D.R.; Trusk, P.B.; Moran, D.M.; Kramer, J.W.; Brown, A.M.; Shell, S.A.; Bacus, S. Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicol. Appl. Pharmacol. 2013, 272, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, S.A.; Heyen, J.R.; Collette, W., III; Bobrowski, W.; Blasi, E.R. Cardiovascular effects in rats following exposure to a receptor tyrosine kinase inhibitor. Toxicol. Pathol. 2010, 38, 416–428. [Google Scholar] [CrossRef]
- Ueda, H.; Nakamura, T.; Matsumoto, K.; Sawa, Y.; Matsuda, H.; Nakamura, T. A potential cardioprotective role of hepatocyte growth factor in myocardial infarction in rats. Cardiovasc. Res. 2001, 51, 41–50. [Google Scholar] [CrossRef]
- Ono, K.; Matsumori, A.; Shioi, T.; Furukawa, Y.; Sasayama, S. Enhanced expression of hepatocyte growth factor c-Met by myocardial ischemia and reperfusion in a rat model. Circulation 1997, 95, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, A.; Furukawa, Y.; Hashimoto, T.; Ono, K.; Shioi, T.; Okada, M.; Iwasaki, A.; Nishio, R.; Sasayama, S. Increased circulating hepatocyte growth factor in the early stage of acute myocardial infarction. Biochem. Biophys. Res. Commun. 1996, 221, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mizuno, S.; Matsumoto, K.; Sawa, Y.; Matsuda, H.; Nakamura, T. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J. Clin. Invest. 2000, 106, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Sala, V.; Crepaldi, T. Novel therapy for myocardial infarction: Can HGF/Met be beneficial? Cell. Mol. Life Sci. 2011, 68, 1703–1717. [Google Scholar] [CrossRef]
- Taylor, C.T.; Pouyssegur, J. Oxygen, hypoxia, and stress. Ann. N. Y. Acad. Sci. 2007, 1113, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kitta, K.; Day, R.M.; Ikeda, T.; Suzuki, Y.J. Hepatocyte growth factor protects cardiac myocytes against oxidative stress-induced apoptosis. Free Radic. Biol. Med. 2001, 31, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Ahmad, N.; Wani, M.A.; Ashraf, M. Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J. Mol. Cell. Cardiol. 2004, 37, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Gatti, S.; Sala, V.; Albano, R.; Costelli, P.; Casanova, E.; Comoglio, P.M.; Crepaldi, T. Agonist antibodies activating the Met receptor protect cardiomyoblasts from cobalt chloride-induced apoptosis and autophagy. Cell Death Dis. 2014, 5, e1185. [Google Scholar] [CrossRef] [PubMed]
- Pietronave, S.; Forte, G.; Locarno, D.; Merlin, S.; Zamperone, A.; Nicotra, G.; Isidoro, C.; di Nardo, P.; Prat, M. Agonist monoclonal antibodies against HGF receptor protect cardiac muscle cells from apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1155–H1165. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Takagi, H.; Qu, X.P.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion—Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Bussolino, F.; Direnzo, M.F.; Ziche, M.; Bocchietto, E.; Olivero, M.; Naldini, L.; Gaudino, G.; Tamagnone, L.; Coffer, A.; Comoglio, P.M. Hepatocyte growth-factor is a potent angiogenic factor which stimulates endothelial-cell motility and growth. J. Cell Biol. 1992, 119, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Merkulova-Rainon, T.; Han, Z.C.; Tobelem, G. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood 2003, 101, 4816–4822. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Morishita, R.; Higaki, J.; Kida, I.; Aoki, M.; Moriguchi, A.; Yamada, K.; Hayashi, S.; Yo, Y.; Nakano, H.; et al. Hepatocyte growth factor is a novel member of the endothelium-specific growth factors: Additive stimulatory effect of hepatocyte growth factor with basic fibroblast growth factor but not with vascular endothelial growth factor. J. Hypertens. 1996, 14, 1067–1072. [Google Scholar] [CrossRef]
- Hayashi, S.; Morishita, R.; Nakamura, S.; Yamamoto, K.; Moriguchi, A.; Nagano, T.; Taiji, M.; Noguchi, H.; Matsumoto, K.; Nakamura, T.; et al. Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease—Down-regulation of HGF in response to hypoxia in vascular cells. Circulation 1999, 100, 301–308. [Google Scholar] [CrossRef]
- Morishita, R.; Nakamura, S.; Hayashi, S.; Taniyama, Y.; Moriguchi, A.; Nagano, T.; Taiji, M.; Noguchi, H.; Takeshita, S.; Matsumoto, K.; et al. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension 1999, 33, 1379–1384. [Google Scholar] [CrossRef]
- Taniyama, Y.; Morishita, R.; Hiraoka, K.; Aoki, M.; Nakagami, H.; Yamasaki, K.; Matsumoto, K.; Nakamura, T.; Kaneda, Y.; Ogihara, T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model—Molecular mechanisms of delayed angiogenesis in diabetes. Circulation 2001, 104, 2344–2350. [Google Scholar] [CrossRef]
- Aoki, M.; Morishita, R.; Taniyama, Y.; Kida, I.; Moriguchi, A.; Matsumoto, K.; Nakamura, T.; Kaneda, Y.; Higaki, J.; Ogihara, T. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: Up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther. 2000, 7, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Riess, I.; Sala, V.; Leo, C.; Demaria, M.; Gatti, S.; Gallo, S.; Fitou, A.; Boero, O.; Levi, R.; Cuccovillo, I.; et al. A mouse model for spatial and temporal expression of HGF in the heart. Transgenic Res. 2011, 20, 1203–1216. [Google Scholar] [CrossRef]
- Jayasankar, V.; Woo, Y.J.; Bish, L.T.; Pirolli, T.J.; Chatterjee, S.; Berry, M.F.; Burdick, J.; Gardner, T.J.; Sweeney, H.L. Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation 2003, 108, II230–II236. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, N.; Takayama, H.; Toyoda, M.; Otsuka, T.; Fukusato, T.; Merlino, G.; Takagi, H.; Mori, M. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene 2002, 21, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Saucier, C.; Khoury, H.; Lai, K.M.V.; Peschard, P.; Dankort, D.; Naujokas, M.A.; Holash, J.; Yancopoulos, G.D.; Muller, W.J.; Pawson, T.; et al. The Shc adaptor protein is critical for VEGF induction by Met/HGF and ErbB2 receptors and for early onset of tumor angiogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 2345–2350. [Google Scholar] [CrossRef]
- Van Belle, E.; Witzenbichler, B.; Chen, D.H.; Silver, M.; Chang, L.; Schwall, R.; Isner, J.M. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor—The case for paracrine amplification of angiogenesis. Circulation 1998, 97, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Wojta, J.; Kaun, C.; Breuss, J.M.; Koshelnick, Y.; Beckmann, R.; Hattey, E.; Mildner, M.; Weninger, W.; Nakamura, T.; Tschachler, E.; et al. Hepatocyte growth factor increases expression of vascular endothelial growth factor and plasminogen activator inhibitor-1 in human keratinocytes and the vascular endothelial growth factor receptor flk-1 in human endothelial cells. Lab. Invest. 1999, 79, 427–438. [Google Scholar]
- Min, J.K.; Lee, Y.M.; Kim, J.H.; Kim, Y.M.; Kim, S.W.; Lee, S.Y.; Gho, Y.S.; Oh, G.T.; Kwon, Y.G. Hepatocyte growth factor suppresses vascular endothelial growth factor-induced expression of endothelial ICAM-1 and VCAM-1 by inhibiting the nuclear factor-κB pathway. Circ. Res. 2005, 96, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Birukova, A.A.; Alekseeva, E.; Mikaelyan, A.; Birukov, K.G. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J. 2007, 21, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Deindl, E.; Zaruba, M.M.; Brunner, S.; Huber, B.; Mehl, U.; Assmann, G.; Hoefer, I.E.; Mueller-Hoecker, J.; Franz, W.M. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J. 2006, 20, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Debusk, L.M.; Babichev, Y.O.; Dumont, D.J.; Lin, P.C. Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood 2006, 108, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Azuma, J.; Taniyama, Y.; Takeya, Y.; Iekushi, K.; Aoki, M.; Dosaka, N.; Matsumoto, K.; Nakamura, T.; Ogihara, T.; Morishita, R. Angiogenic and antifibrotic actions of hepatocyte growth factor improve cardiac dysfunction in porcine ischemic cardiomyopathy. Gene Ther. 2006, 13, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Minatoguchi, S.; Kosai, K.; Yuge, K.; Takahashi, T.; Arai, M.; Wang, N.Y.; Misao, Y.; Lu, C.J.; Onogi, H.; et al. In vivo hepatocyte growth factor gene transfer reduces myocardial ischemia-reperfusion injury through its multiple actions. J. Card. Fail. 2007, 13, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumoto, K.; Mizuno, S.; Sawa, Y.; Matsuda, H.; Nakamura, T. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2131–H2139. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, Y.; Morishita, R.; Aoki, M.; Hiraoka, K.; Yamasaki, K.; Hashiya, N.; Matsumoto, K.; Nakamura, T.; Kaneda, Y.; Ogihara, T. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Hypertension 2002, 40, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, Y.; Morishita, R.; Nakagami, H.; Moriguchi, A.; Sakonjo, H.; Shokei, K.; Matsumoto, K.; Nakamura, T.; Higaki, J.; Ogihara, T. Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation 2000, 102, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Dai, C.S.; Liu, Y.H. Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am. J. Pathol. 2003, 163, 621–632. [Google Scholar] [CrossRef]
- Kobayashi, E.; Sasamura, H.; Mifune, M.; Shimizu-Hirota, R.; Kuroda, M.; Hayashi, M.; Saruta, T. Hepatocyte growth factor regulates proteoglycan synthesis in interstitial fibroblasts. Kidney Int. 2003, 64, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Matsumoto, K.; Li, M.Y.; Nakamura, T. HGF reduces advancing lung fibrosis in mice: A potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005, 19, 580–582. [Google Scholar] [PubMed]
- Esaki, M.; Takemura, G.; Kosai, K.I.; Takahashi, T.; Miyata, S.; Li, L.H.; Goto, K.; Maruyama, R.; Okada, H.; Kanamori, H.; et al. Treatment with an adenoviral vector encoding hepatocyte growth factor mitigates established cardiac dysfunction in doxorubicin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1048–H1057. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Egashira, K.; Tomita, H.; Usui, M.; Okamoto, H.; Kitabatake, A.; Shimokawa, H.; Sueishi, K.; Takeshita, A. Chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade—Effects on cardiovascular remodeling in rats induced by the long-term blockade of nitric oxide synthesis. Hypertension 1997, 30, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Egashira, K.; Ohara, Y.; Takemoto, M.; Koyanagi, M.; Katoh, M.; Yamamoto, H.; Tamaki, K.; Shimokawa, H.; Takeshita, A. Early induction of transforming growth factor-β via angiotensin II type 1 receptors contributes to cardiac fibrosis induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 1998, 32, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Purdie, K.J.; Whitley, G.S.; Johnstone, A.P.; Cartwright, J.E. Hepatocyte growth factor-induced endothelial cell motility is mediated by the upregulation of inducible nitric oxide synthase expression. Cardiovasc. Res. 2002, 54, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Moriguchi, A.; Morishita, R.; Kida, I.; Tomita, N.; Matsumoto, K.; Nakamura, T.; Higaki, J.; Ogihara, T. Role of angiotensin II in the regulation of a novel vascular modulator, hepatocyte growth factor (HGF), in experimental hypertensive rats. Hypertension 1997, 30, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Morishita, R.; Moriguchi, A.; Nakamura, Y.; Hayashi, S.; Aoki, M.; Kida, I.; Matsumoto, K.; Nakamura, T.; Higaki, J.; et al. Negative regulation of local hepatocyte growth factor expression by angiotensin II and transforming growth factor-beta in blood vessels—Potential role of HGF in cardiovascular disease. Hypertension 1998, 32, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Nakamura, T. Prevention of neutrophil extravasation by hepatocyte growth factor leads to attenuations of tubular apoptosis and renal dysfunction in mouse ischemic kidneys. Am. J. Pathol. 2005, 166, 1895–1905. [Google Scholar] [CrossRef]
- Galimi, F.; Cottone, E.; Vigna, E.; Arena, N.; Boccaccio, C.; Giordano, S.; Naldini, L.; Comoglio, P.M. Hepatocyte growth factor is a regulator of monocyte-macrophage function. J. Immunol. 2001, 166, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Kamimoto, M.; Mizuno, S.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor prevents multiple organ injuries in endotoxemic mice through a heme oxygenase-1-dependent mechanism. Biochem. Biophys. Res. Commun. 2009, 380, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Futamatsu, H.; Suzuki, J.; Mizuno, S.; Koga, N.; Adachi, S.; Kosuge, H.; Maejima, Y.; Hirao, K.; Nakamura, T.; Isobe, M. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis—A potential role for induction of T helper 2 cytokines. Circ. Res. 2005, 96, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Bonanno, G.; Procoli, A.; Mariott, A.; de Ritis, D.G.; Curti, A.; Danese, S.; Pessina, G.; Pandolfi, S.; Natoni, F.; et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood 2006, 108, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Benkhoucha, M.; Santiago-Raber, M.L.; Schneiter, G.; Chofflon, M.; Funakoshi, H.; Nakamura, T.; Lalive, P.H. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2010, 107, 6424–6429. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Xu, S.L.; Chen, B.; Zhang, S.L.; Zhang, Y.L.; Wei, W.; Ma, D.C.; Wang, L.S.; Zhu, T.B.; Li, C.J.; et al. Hepatocyte growth factor plays a critical role in the regulation of cytokine production and induction of endothelial progenitor cell mobilization: A pilot gene therapy study in patients with coronary heart disease. Clin. Exp. Pharmacol. Physiol. 2009, 36, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Besnard, S.; Duriez, M.; Deleuze, V.; Emmanuel, F.; Bureau, M.F.; Soubrier, F.; Esposito, B.; Duez, H.; Fievet, C.; et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 1999, 85, E17–E24. [Google Scholar] [CrossRef] [PubMed]
- Mtairag, E.; Chollet-Martin, S.; Oudghiri, M.; Laquay, N.; Jacob, M.P.; Michel, J.B.; Feldman, L.J. Effects of interleukin-10 on monocyte/endothelial cell adhesion and MMP-9/TIMP-1 secretion. Cardiovasc. Res. 2001, 49, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.L.; Wang, X.; Sung, C.P.; Olson, B.; Mckenna, P.J.; Gu, J.L.; Feuerstein, G.Z. Interleukin-8—A mitogen and chemoattractant for vascular smooth-muscle cells. Circ. Res. 1994, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, R.E.; Garcia-Zepeda, E.A.; Lim, Y.C.; Yoshida, M.; Ding, H.A.; Gimbrone, M.A.; Luster, A.D.; Luscinskas, F.W.; Rosenzweig, A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999, 398, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, K.; Ito, K.; Tsukioka, K.; Wada, Y.; Makiuchi, A.; Sakaguchi, M.; Akashima, T.; Fujimori, M.; Sawa, Y.; Morishita, R.; et al. Suppression of acute and chronic rejection by hepatocyte growth factor in a murine model of cardiac transplantation—Induction of tolerance and prevention of cardiac allograft vasculopathy. Circulation 2004, 110, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Kim, Y.J.; Han, S.H.; Kang, C.Y. IFN-gamma-STAT1 signal regulates the differentiation of inducible Treg: Potential role for ROS-mediated apoptosis. Eur. J. Immunol. 2009, 39, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.D.; Guerquin-Kern, J.L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, M.L.; Lee, R.T. Regeneration of the heart. EMBO Mol. Med. 2011, 3, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Ellison, G.M.; Torella, D.; Dellegrottaglie, S.; Perez-Martinez, C.; de Prado, A.P.; Vicinanza, C.; Purushothaman, S.; Galuppo, V.; Iaconetti, C.; Waring, C.D.; et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J. Am. Coll. Cardiol. 2011, 58, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Torella, D.; Ellison, G.M.; Mendez-Ferrer, S.; Ibanez, B.; Nadal-Ginard, B. Resident human cardiac stem cells: Role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, S8–S13. [Google Scholar] [CrossRef] [PubMed]
- Laugwitz, K.L.; Moretti, A.; Lam, J.; Gruber, P.; Chen, Y.H.; Woodard, S.; Lin, L.Z.; Cai, C.L.; Lu, M.M.; Reth, M.; et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005, 433, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Quaini, F.; Urbanek, K.; Beltrami, A.P.; Finato, N.; Beltrami, C.A.; Nadal-Ginard, B.; Kajstura, J.; Leri, A.; Anversa, P. Chimerism of the transplanted heart. N. Engl. J. Med. 2002, 346, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Messina, E.; de Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.G.; Coletta, M.; et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michael, L.H.; Behringer, R.R.; Garry, D.J.; et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318. [Google Scholar] [CrossRef] [PubMed]
- Stastna, M.; Abraham, M.R.; van Eyk, J.E. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett. 2009, 583, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Stastna, M.; Chimenti, I.; Marban, E.; van Eyk, J.E. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics 2010, 10, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, K.; Rota, M.; Cascapera, S.; Bearzi, C.; Nascimbene, A.; de Angelis, A.; Hosoda, T.; Chimenti, S.; Baker, M.; Limana, F.; et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ. Res. 2005, 97, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, I.; Smith, R.R.; Li, T.S.; Gerstenblith, G.; Messina, E.; Giacomello, A.; Marban, E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 2010, 106, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Muller, P.; Nurzynska, D.; Casarsa, C.; Torella, D.; Nascimbene, A.; Castaldo, C.; Cascapera, S.; Bohm, M.; Quaini, F.; et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc. Natl. Acad. Sci. USA 2005, 102, 8966–8971. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.; Padin-Iruegas, M.E.; Misao, Y.; de Angelis, A.; Maestroni, S.; Ferreira-Martins, J.; Fiumana, E.; Rastaldo, R.; Arcarese, M.L.; Mitchell, T.S.; et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ. Res. 2008, 103, 107–116. [Google Scholar] [CrossRef]
- Smart, N.; Risebro, C.A.; Melville, A.A.D.; Moses, K.; Schwartz, R.J.; Chien, K.R.; Riley, P.R. Thymosin β4 induces adult epicardial progenitor mobilization and neovascularization. Nature 2007, 445, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Limana, F.; Zacheo, A.; Mocini, D.; Mangoni, A.; Borsellino, G.; Diamantini, A.; de Mori, R.; Battistini, L.; Vigna, E.; Santini, M.; et al. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ. Res. 2007, 101, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Limana, F.; Bertolami, C.; Mangoni, A.; di Carlo, A.; Avitabile, D.; Mocini, D.; Iannelli, P.; de Mori, R.; Marchetti, C.; Pozzoli, O.; et al. Myocardial infarction induces embryonic reprogramming of epicardial c-kit+ cells: Role of the pericardial fluid. J. Mol. Cell. Cardiol. 2010, 48, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.; Koller, L.; Hohensinner, P.J.; Zorn, G.; Brekalo, M.; Berger, R.; Mörtl, D.; Maurer, G.; Pacher, R.; Huber, K.; et al. A multi-biomarker risk score improves prediction of long-term mortality in patients with advanced heart failure. Int. J. Cardiol. 2013, 168, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Rychli, K.; Richter, B.; Hohensinner, P.J.; Kariem Mahdy, A.; Neuhold, S.; Zorn, G.; Berger, R.; Mörtl, D.; Huber, K.; Pacher, R.; et al. Hepatocyte growth factor is a strong predictor of mortality in patients with advanced heart failure. Heart 2011, 97, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Morishita, R.; Moriguchi, A.; Higaki, J.; Ogihara, T. Hepa-tocyte growth factor (HGF) as a potential index of severity of hypertension. Hypertens. Res. Clin. Exp. 1999, 22, 161–167. [Google Scholar]
- Nakamura, S.; Morishita, R.; Moriguchi, A.; Yo, Y.; Nakamura, Y.; Hayashi, S.; Matsumoto, K.; Matsumoto, K.; Nakamura, T.; Higaki, J.; et al. Hepatocyte growth factor as a potential indexof complication in diabetes mellitus. J. Hypertens. 1998, 16, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Kämppä, N.; Mäkelä, K.M.; Lyytikäinen, L.P.; Peltonen, N.; Hautamäki, J.; Seppälä, I.; Mononen, N.; Goebeler, S.; Karhunen, P.J.; Hervonen, A.; et al. Vascular cell adhesion molecule 1, soluble Fas and hepatocyte growth factor as predictors of mortality in nonagenarians: The vitality 90+ study. Exp. Gerontol. 2013, 48, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Sorour, A.E.; Lönn, J.; Nakka, S.S.; Nayeri, T.; Nayeri, F. Evaluation of hepatocyte growth factor as a local acute phase response marker in the bowel: The clinical impact of a rapid diagnostic test for immediate identification of acute bowel inflammation. Cytokine 2014, 71, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B 2010, 86, 588–610. [Google Scholar] [CrossRef]
- Cassano, M.; Biressi, S.; Finan, A.; Benedetti, L.; Omes, C.; Boratto, R.; Martin, F.; Allegretti, M.; Broccoli, V.; de Angelis, G.C.; et al. Magic-factor 1, a partial agonist of Met, induces muscle hypertrophy by protecting myogenic progenitors from apoptosis. PLoS One 2008, 3, e3223. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Tsai, P.C.; Cochran, J.R. Engineering hepatocyte growth factor fragments with high stability and activity as Met receptor agonists and antagonists. Proc. Natl. Acad. Sci. USA 2011, 108, 13035–13040. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.H. Structural basis of MET receptor dimerization by the bacterial invasion protein InlB and the HGF/SF splice variant NK1. Biochim. Biophys. Acta 2013, 1834, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Prat, M.; Crepaldi, T.; Pennacchietti, S.; Bussolino, F.; Comoglio, P.M. Agonistic monoclonal antibodies against the Met receptor dissect the biological responses to HGF. J. Cell Sci. 1998, 111, 237–247. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, S.; Sala, V.; Gatti, S.; Crepaldi, T. HGF/Met Axis in Heart Function and Cardioprotection. Biomedicines 2014, 2, 247-262. https://doi.org/10.3390/biomedicines2040247
Gallo S, Sala V, Gatti S, Crepaldi T. HGF/Met Axis in Heart Function and Cardioprotection. Biomedicines. 2014; 2(4):247-262. https://doi.org/10.3390/biomedicines2040247
Chicago/Turabian StyleGallo, Simona, Valentina Sala, Stefano Gatti, and Tiziana Crepaldi. 2014. "HGF/Met Axis in Heart Function and Cardioprotection" Biomedicines 2, no. 4: 247-262. https://doi.org/10.3390/biomedicines2040247
APA StyleGallo, S., Sala, V., Gatti, S., & Crepaldi, T. (2014). HGF/Met Axis in Heart Function and Cardioprotection. Biomedicines, 2(4), 247-262. https://doi.org/10.3390/biomedicines2040247