HGF/Met-Signaling Contributes to Immune Regulation by Modulating Tolerogenic and Motogenic Properties of Dendritic Cells
Abstract
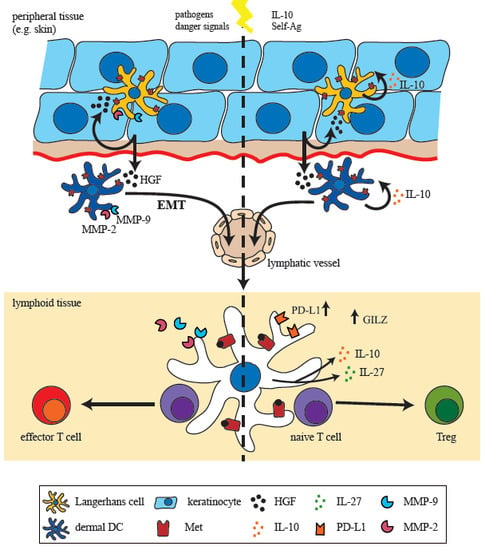
:1. Introduction on the Immunoregulatory Function of HGF/Met
2. Dendritic Cells Are Key Regulators of the Immune System
3. HGF Mediates Development of Tolerogenic Dendritic Cells (DCs)
4. Met-Signaling Impacts on DC Migration
4.1. Met-Signaling in Monocytes
4.2. Met-Signaling in DC Motility and Migration
5. A Met-Driven Program of Epithelial-Mesenchymal Transition in DCs?
6. Conclusions and Perspectives

Acknowledgments
Conflicts of Interest
References
- Park, M.; Dean, M.; Cooper, C.S.; Schmidt, M.; O’Brien, S.J.; Blair, D.G.; vande Woude, G.F. Mechanism of met oncogene activation. Cell 1986, 45, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Teramoto, H.; Ichihara, A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc. Natl. Acad. Sci. USA 1986, 83, 6489–6493. [Google Scholar] [CrossRef] [PubMed]
- Stoker, M.; Gherardi, E.; Perryman, M.; Gray, J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 1987, 327, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Weidner, K.M.; Arakaki, N.; Hartmann, G.; Vandekerckhove, J.; Weingart, S.; Rieder, H.; Fonatsch, C.; Tsubouchi, H.; Hishida, T.; Daikuhara, Y.; et al. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc. Natl. Acad. Sci. USA 1991, 88, 7001–7005. [Google Scholar] [CrossRef]
- Sonnenberg, E.; Meyer, D.; Weidner, K.M.; Birchmeier, C. Scatter factor/hepatocyte growth factor and its receptor, the c-Met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J. Cell Biol. 1993, 123, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Bladt, F.; Riethmacher, D.; Isenmann, S.; Aguzzi, A.; Birchmeier, C. Essential role for the c-Met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995, 376, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Bladt, F.; Goedecke, S.; Brinkmann, V.; Zschiesche, W.; Sharpe, M.; Gherardi, E.; Birchmeier, C. Scatter factor/hepatocyte growth-factor is essential for liver development. Nature 1995, 373, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Minowa, O.; Mori, C.; Shiota, K.; Kuno, J.; Noda, T.; Kitamura, N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995, 373, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Block, G.D.; Locker, J.; Bowen, W.C.; Petersen, B.E.; Katyal, S.; Strom, S.C.; Riley, T.; Howard, T.A.; Michalopoulos, G.K. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J. Cell Biol. 1996, 132, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Borowiak, M.; Garratt, A.N.; Wustefeld, T.; Strehle, M.; Trautwein, C.; Birchmeier, C. Met provides essential signals for liver regeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 10608–10613. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Perez, J.C.; Ernst, S.; Demirci, C.; Casinelli, G.P.; Mellado-Gil, J.M.; Rausell-Palamos, F.; Vasavada, R.C.; Garcia-Ocana, A. Hepatocyte growth factor/c-Met signaling is required for beta-cell regeneration. Diabetes 2014, 63, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, F.; Xu, J.; Abdiu, A.; Nayeri, T.; Aili, D.; Liedberg, B.; Carlsson, U. Autocrine production of biologically active hepatocyte growth factor (HGF) by injured human skin. J. Dermatol. Sci. 2006, 43, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chmielowiec, J.; Borowiak, M.; Morkel, M.; Stradal, T.; Munz, B.; Werner, S.; Wehland, J.; Birchmeier, C.; Birchmeier, W. c-Met is essential for wound healing in the skin. J. Cell Biol. 2007, 177, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Cowin, A.J.; Kallincos, N.; Hatzirodos, N.; Robertson, J.G.; Pickering, K.J.; Couper, J.; Belford, D.A. Hepatocyte growth factor and macrophage-stimulating protein are upregulated during excisional wound repair in rats. Cell Tissue Res. 2001, 306, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Birchmeier, C.; Birchmeier, W.; Gherardi, E.; vande Woude, G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Boccaccio, C.; Comoglio, P.M. Invasive growth: A Met-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer 2006, 6, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Bodescot, M.; Blair, D.; Dunn, J.; Nakamura, T.; Mizuno, K.; Park, M.; Chan, A.; Aaronson, S.; vande Woude, G.F. Tumorigenicity of the Met proto-oncogene and the gene for hepatocyte growth factor. Mol. Cell. Biol. 1992, 12, 5152–5158. [Google Scholar] [PubMed]
- Kanda, H.; Tajima, H.; Lee, G.H.; Nomura, K.; Ohtake, K.; Matsumoto, K.; Nakamura, T.; Kitagawa, T. Hepatocyte growth factor transforms immortalized mouse liver epithelial cells. Oncogene 1993, 8, 3047–3053. [Google Scholar] [PubMed]
- Nakamura, T.; Sakai, K.; Nakamura, T.; Matsumoto, K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J. Gastroen. Hepatol. 2011, 26, 188–202. [Google Scholar] [CrossRef]
- Jiang, W.G.; Martin, T.A.; Parr, C.; Davies, G.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit. Rev. Oncol. Hematol. 2005, 53, 35–69. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Sciacchitano, S.; Vitale, A.; Cardelli, P.; Galletti, M.; Vitarelli, E.; Barresi, G.; Benvenga, S.; Trimarchi, F.; Trovato, M. Serum hepatocyte growth factor is increased in hashimoto’s thyroiditis whether or not it is associated with nodular goiter as compared with healthy non-goitrous individuals. J. Endocrinol. Investig. 2009, 32, 465–469. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Vitarelli, E.; Barresi, G.; Trimarchi, F.; Benvenga, S.; Trovato, M. The tyrosine kinase receptor c-met, its cognate ligand HGF and the tyrosine kinase receptor trasducers STAT3, PI3K and RHO in thyroid nodules associated with Hashimoto’s thyroiditis: An immunohistochemical characterization. Eur. J. Histochem. 2010, 54, e24. [Google Scholar] [CrossRef] [PubMed]
- Beilmann, M.; Odenthal, M.; Jung, W.; vande Woude, G.F.; Dienes, H.P.; Schirmacher, P. Neoexpression of the c-Met/hepatocyte growth factor-scatter factor receptor gene in activated monocytes. Blood 1997, 90, 4450–4458. [Google Scholar] [PubMed]
- Van der Voort, R.; Taher, T.E.; Keehnen, R.M.; Smit, L.; Groenink, M.; Pals, S.T. Paracrine regulation of germinal center B cell adhesion through the c-met-hepatocyte growth factor/scatter factor pathway. J. Exp. Med. 1997, 185, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Benkhoucha, M.; Santiago-Raber, M.L.; Schneiter, G.; Chofflon, M.; Funakoshi, H.; Nakamura, T.; Lalive, P.H. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2010, 107, 6424–6429. [Google Scholar] [CrossRef] [PubMed]
- Okunishi, K.; Dohi, M.; Nakagome, K.; Tanaka, R.; Mizuno, S.; Matsumoto, K.; Miyazaki, J.; Nakamura, T.; Yamamoto, K. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J. Immunol. 2005, 175, 4745–4753. [Google Scholar] [CrossRef] [PubMed]
- Kurz, S.M.; Diebold, S.S.; Hieronymus, T.; Gust, T.C.; Bartunek, P.; Sachs, M.; Birchmeier, W.; Zenke, M. The impact of c-Met/scatter factor receptor on dendritic cell migration. Eur. J. Immunol. 2002, 32, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Birchmeier, C.; Zenke, M.; Hieronymus, T. The HGF receptor/Met tyrosine kinase is a key regulator of dendritic cell migration in skin immunity. J. Immunol. 2012, 189, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.; Vollmann, E.H.; von Andrian, U.H. Mechanisms and consequences of dendritic cell migration. Immunity 2008, 29, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Stoitzner, P.; Tripp, C.H.; Eberhart, A.; Price, K.M.; Jung, J.Y.; Bursch, L.; Ronchese, F.; Romani, N. Langerhans cells cross-present antigen derived from skin. Proc. Natl. Acad. Sci. USA 2006, 103, 7783–7788. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, S.; Scholz, C.; Kautz, A.; Tampe, R.; Kurts, C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat. Immunol. 2008, 9, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Sznol, M.; Zhao, B.; Wang, D.; Carvajal, R.D.; Keohan, M.L.; Chuang, E.; Sanborn, R.E.; Lutzky, J.; Powderly, J.; et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci. Transl. Med. 2014, 6, 232ra251. [Google Scholar] [CrossRef]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Singhal, E.; Sen, P. Hepatocyte growth factor-induced c-Src-phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway inhibits dendritic cell activation by blocking IkappaB kinase activity. Int. J. Biochem. Cell Biol. 2011, 43, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Corinti, S.; Albanesi, C.; la Sala, A.; Pastore, S.; Girolomoni, G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 2001, 166, 4312–4318. [Google Scholar] [CrossRef] [PubMed]
- Singhal, E.; Kumar, P.; Sen, P. A novel role for bruton’s tyrosine kinase in hepatocyte growth factor-mediated immunoregulation of dendritic cells. J. Biol. Chem. 2011, 286, 32054–32063. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Bonanno, G.; Procoli, A.; Mariotti, A.; de Ritis, D.G.; Curti, A.; Danese, S.; Pessina, G.; Pandolfi, S.; Natoni, F.; et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood 2006, 108, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Benkhoucha, M.; Molnarfi, N.; Dunand-Sauthier, I.; Merkler, D.; Schneiter, G.; Bruscoli, S.; Riccardi, C.; Tabata, Y.; Funakoshi, H.; Nakamura, T.; et al. Hepatocyte growth factor limits autoimmune neuroinflammation via glucocorticoid-induced leucine zipper expression in dendritic cells. J. Immunol. 2014, 193, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Ayroldi, E.; Migliorati, G.; Bruscoli, S.; Marchetti, C.; Zollo, O.; Cannarile, L.; D’Adamio, F.; Riccardi, C. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood 2001, 98, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Mouly, E.; Hamdi, H.; Maillot, M.C.; Pallardy, M.; Godot, V.; Capel, F.; Balian, A.; Naveau, S.; Galanaud, P.; et al. Gilz expression in human dendritic cells redirects their maturation and prevents antigen-specific T lymphocyte response. Blood 2006, 107, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, H.; Godot, V.; Maillot, M.C.; Prejean, M.V.; Cohen, N.; Krzysiek, R.; Lemoine, F.M.; Zou, W.; Emilie, D. Induction of antigen-specific regulatory T lymphocytes by human dendritic cells expressing the glucocorticoid-induced leucine zipper. Blood 2007, 110, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; DeFrances, M.C.; Zarnegar, R. Induction of met proto-oncogene (hepatocyte growth factor receptor) expression during human monocyte-macrophage differentiation. Cell Growth Differ. 1996, 7, 821–832. [Google Scholar] [PubMed]
- Beilmann, M.; Vande Woude, G.F.; Dienes, H.P.; Schirmacher, P. Hepatocyte growth factor-stimulated invasiveness of monocytes. Blood 2000, 95, 3964–3969. [Google Scholar] [PubMed]
- Moransard, M.; Sawitzky, M.; Fontana, A.; Suter, T. Expression of the HGF receptor c-Met by macrophages in experimental autoimmune encephalomyelitis. Glia 2010, 58, 559–571. [Google Scholar] [PubMed]
- Galimi, F.; Cottone, E.; Vigna, E.; Arena, N.; Boccaccio, C.; Giordano, S.; Naldini, L.; Comoglio, P.M. Hepatocyte growth factor is a regulator of monocyte-macrophage function. J. Immunol. 2001, 166, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Ratzinger, G.; Stoitzner, P.; Ebner, S.; Lutz, M.B.; Layton, G.T.; Rainer, C.; Senior, R.M.; Shipley, J.M.; Fritsch, P.; Schuler, G.; et al. Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J. Immunol. 2002, 168, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.H.; Khayrullina, T.; Ganea, D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood 2008, 111, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Saalbach, A.; Klein, C.; Schirmer, C.; Briest, W.; Anderegg, U.; Simon, J.C. Dermal fibroblasts promote the migration of dendritic cells. J. Investig. Dermatol. 2010, 130, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.T.; Limb, G.A.; Saarialho-Kere, U.; Murphy, G.; Khaw, P.T. Human corneal epithelial cells require MMP-1 for HGF-mediated migration on collagen I. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1048–1055. [Google Scholar] [CrossRef]
- McCawley, L.J.; O’Brien, P.; Hudson, L.G. Epidermal growth factor (EGF)- and scatter factor/hepatocyte growth factor (SF/HGF)-mediated keratinocyte migration is coincident with induction of matrix metalloproteinase (MMP)-9. J. Cell. Physiol. 1998, 176, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Comoglio, P.M. Scatter-factor and semaphorin receptors: Cell signalling for invasive growth. Nat. Rev. Cancer 2002, 2, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Christofori, G. New signals from the invasive front. Nature 2006, 441, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Hieronymus, T.; Zenke, M.; Baek, J.H.; Sere, K. The clash of Langerhans cell homeostasis in skin: Should I stay or should I go? Semin. Cell Dev. Biol. 2014. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Iwasaki, T.; Imado, T.; Sekiguchi, M.; Fujimoto, J.; Sano, H. Hepatocyte growth factor prevents lupus nephritis in a murine lupus model of chronic graft-versus-host disease. Arthritis Res. Ther. 2006, 8, R123. [Google Scholar] [CrossRef] [PubMed]
- Okunishi, K.; Dohi, M.; Fujio, K.; Nakagome, K.; Tabata, Y.; Okasora, T.; Seki, M.; Shibuya, M.; Imamura, M.; Harada, H.; et al. Hepatocyte growth factor significantly suppresses collagen-induced arthritis in mice. J. Immunol. 2007, 179, 5504–5513. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Nascimento, J.M.; Kowar, S.; Busch, H.; Boerries, M. Boolean approach to signalling pathway modelling in HGF-induced keratinocyte migration. Bioinformatics 2012, 28, i495–i501. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hübel, J.; Hieronymus, T. HGF/Met-Signaling Contributes to Immune Regulation by Modulating Tolerogenic and Motogenic Properties of Dendritic Cells. Biomedicines 2015, 3, 138-148. https://doi.org/10.3390/biomedicines3010138
Hübel J, Hieronymus T. HGF/Met-Signaling Contributes to Immune Regulation by Modulating Tolerogenic and Motogenic Properties of Dendritic Cells. Biomedicines. 2015; 3(1):138-148. https://doi.org/10.3390/biomedicines3010138
Chicago/Turabian StyleHübel, Jessica, and Thomas Hieronymus. 2015. "HGF/Met-Signaling Contributes to Immune Regulation by Modulating Tolerogenic and Motogenic Properties of Dendritic Cells" Biomedicines 3, no. 1: 138-148. https://doi.org/10.3390/biomedicines3010138
APA StyleHübel, J., & Hieronymus, T. (2015). HGF/Met-Signaling Contributes to Immune Regulation by Modulating Tolerogenic and Motogenic Properties of Dendritic Cells. Biomedicines, 3(1), 138-148. https://doi.org/10.3390/biomedicines3010138