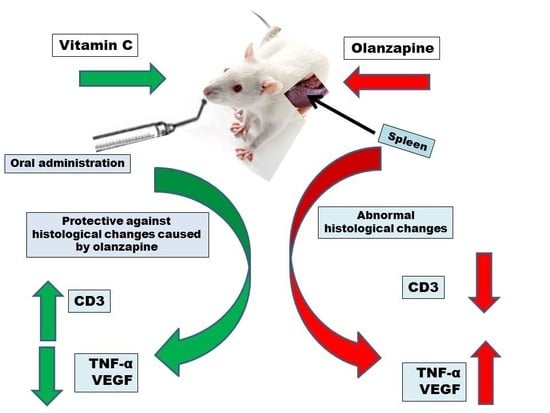
Differential Expression of CD3, TNF-α, and VEGF Induced by Olanzapine on the Spleen of Adult Male Albino Rats and the Possible Protective Role of Vitamin C
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Housing
2.2. Chemicals
2.3. Experimental Design
2.4. Histological Study
2.5. Immunohistochemistry
2.6. Morphometric Study
2.7. Statistical Analysis
3. Results
3.1. H&E Results
3.2. CD3 Immunohistochemistry and Morphometric Results
3.3. TNF-α and VEGF Immunohistochemistry and Morphometric Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Todorović, N.; Tomanović, N.; Gass, P.; Filipović, D. Olanzapine modulation of hepatic oxidative stress and inflammation in socially isolated rats. Eur. J. Pharm. Sci. 2016, 81, 94–102. [Google Scholar] [CrossRef]
- Elbakary, R.A. Histological Study of the Effects of Olanzapine on the Liver of Adult Male Albino Rat with and without Vitamin, C. Egypt. J. Histol. 2017, 40, 1–11. [Google Scholar] [CrossRef]
- Chawla, N.; Kuma, S.; Balhara, Y.P.S. Olanzapine-induced Skin Eruptions. Indian J. Psychol. Med. 2017, 39, 537–538. [Google Scholar]
- Domínguez-Jiménez, J.L.; Puente-Gutiérrez, J.J.; Pelado-García, E.M.; Cuesta-Cubillas, D.; García-Moreno, A.M. Liver toxicity due to olanzapine. Rev. Esp. Enferm. Dig. 2012, 104, 617–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houseknecht, K.L.; Robertson, A.S.; Zavadoski, W.; Gibbs, E.M.; Johnson, D.E.; Rollema, H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: Implications for adverse metabolic effects. Neuropsychopharmacology 2006, 32, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Weston-Green, K.; Huang, X.F.; Deng, C. Olanzapine treatment and metabolic dysfunction: A dose response study in female Sprague Dawley rats. Behav. Brain Res. 2011, 217, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.C.; Mohanty, B. Effects of lactational exposure of olanzapine and risperidone on hematology and lymphoid organs histopathology: A comparative study in mice neonates. Eur. J. Pharm. 2010, 634, 170–177. [Google Scholar] [CrossRef]
- Walss-Bass, C.; Weintraub, S.T.; Hatch, J. Clozapine causes oxidation of proteins involved in energy metabolism: A possible mechanism for antipsychotic induced metabolic alterations. Int. J. Neuropsychopharmacol. 2008, 11, 1097–1104. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.P.; Redasani, V.K.; Kalaskar, M.G. Protective effect of Ginkgo biloba on ethanol-induced immunosuppression in rats. Eur. J. Exp. Biol. 2011, 1, 83–89. [Google Scholar]
- Liu, J.; Wang, H.; Zhao, W. Induction of pathological changes and impaired expression of cytokines in developing female rat spleen after chronic excess fluoride exposure. Toxicol. Ind. Health 2019, 35, 43–52. [Google Scholar] [CrossRef]
- Mohamed, D.S.; Abdelhaliem, N.G.; Zakaria, A.M. Histological and Immunohistochemical Study of the Possible Protective Effect of Ascorbic Acid on the Toxic Effect of Monosodium Glutamate on the Spleen of Adult Male Albino Rat. Egypt. J. Histol. 2017, 40, 94–104. [Google Scholar] [CrossRef]
- Ozmen, O. Endosulfan splenic pathology and amelioration by vitamin C in New Zealand rabbit. J. Immunotoxicol. 2016, 13, 349–354. [Google Scholar] [CrossRef]
- Ströhle, A.; Wolters, M.; Hahn, A. Micronutrients at the Interface Between Inflammation and Infection Ascorbic Acid and Calciferol. Part 1: General Overview with a Focus on Ascorbic Acid. Inflamm. Allergy Drug Targets 2011, 10, 54–63. [Google Scholar]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Immune enhancing role of Vitamin C and zinc and effect on clinical conditions. Nutr. Metab. 2006, 50, 85–94. [Google Scholar] [CrossRef]
- Sang, X.Z.; Zheng, L.; Sun, Q.Q.; Zhang, T.; Li, N.; Cui, Y.; Hu, R.; Gao, G.; Cheng, Z.; Cheng, J.; et al. The chronic spleen injury of mice following exposure to titanium dioxide nanoparticules. J. Biomed. Mater. Res. 2012, 100, 894–902. [Google Scholar] [CrossRef]
- Rahman, H.; Aman Upaganlawar, A.; Upasani, C. Protective Effect of Ferulic Acid Alone and in Combination with Ascorbic Acid on Aniline Induced Spleen Toxicity. Ann. Pharmacol. Pharm. 2017, 2, 1–5. [Google Scholar]
- Paget, G.E.; Barnes, J.M. Evaluation of Drug Activities and Pharmacometrics; Academic Press: Cambridge, MA, USA, 1964; p. 135. [Google Scholar]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques, 7th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; Staining methods; pp. 147–150, 263–325. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; Immunohistochemical techniques; pp. 433–472. [Google Scholar]
- Basta-Kaim, A.; Kubera, M.; Budziszewska, B.; Siwanowicz, J.; Lasoñ, W. Effect of some antipsychotic drugs on immunoreactivity in C57BL/6 mice. Pol. J. Pharm. 2002, 54, 737–742. [Google Scholar]
- El-Awdan, S.A.; Abdel-Sala, O.M. Modulation of Antipsychotic-induced Oxidative Stress by Selective and Non Selective COX2 Nonsteroidal Anti-inflammatory Drugs. Pharmacologia 2012, 3, 222–226. [Google Scholar] [CrossRef]
- Victoriano, M.; de Beaurepaire, R.; Naour, N.; Guerre-Millo, M.; Quignard-Boulangé, A.; Huneau, J.F.; Mathé, V.; Tomé, D.; Hermier, D. Olanzapine-induced accumulation of adipose tissue is associated with an inflammatory state. Brain Res. 2010, 1350, 167–175. [Google Scholar] [CrossRef]
- Eftekhari, A.; Azarmi, Y.; Parvizpur, A.; Eghbal, M.A. Involvement of oxidative stress and mitochondrial/lysosomal cross-talk in Olanzapine cytotoxicity in freshly isolated rat hepatocytes. Xenobiotica 2016, 46, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Isaacs-Trepanier, C.; Elmi, N.; Rapoport, S.I.; Andreazza, A.C. Mitochondrial dysfunction and lipid peroxidation in rat frontal cortex by chronic NMDA administration can be partially prevented by lithium treatment. J. Psychiatr. Res. 2016, 76, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mazen, N.F.; Saleh, E.Z.; Mahmoud, A.A.; Shaalan, A.A. Histological and immunohistochemical study on the potential toxicity of sliver nanoparticles on the structure of the spleen in adult male albino rats. Egypt. J. Histol. 2017, 40, 374–387. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Choi, Y.J.; Han, J.W.; Kim, E.; Park, J.H.; Gurunathan, S.; Kim, J.H. Differential nanoreprotoxicity of silver nanoparticles in male somatic cells and spermatogonial stem cells. Int. J. Nanomed. 2015, 10, 1335–1357. [Google Scholar]
- Cesta, M. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Parola, M. oxidative damage and fibrogenesis. Free Radic. Biol. Med. 2006, 22, 287–305. [Google Scholar] [CrossRef]
- Ayuob, N.N. Can vitamin E and selenium alleviate the immunologic impact of aluminium on pregnant rats’ spleens? Cell Immunol. 2013, 284, 104–110. [Google Scholar] [CrossRef]
- Hassan, Z.A.; Arafa, M.H.; Soliman, W.I.; Atteia, H.H.; Al-Saeed, H.F. The Effects of Monosodium Glutamate on Thymic and Splenic Immune Functions and Role of Recovery (Biochemical and Histological study). J. Cytol. Histol. 2014, 5, 283. [Google Scholar] [CrossRef]
- Zhang, Q.; He, M.; Deng, C.; Wang, H.; Huang, X.F. Effects of olanzapine on the elevation of macrophage. infiltration and pro-inflammatory cytokine expression in female rats. J. Psychopharmacol. 2014, 28, 1161–1169. [Google Scholar] [CrossRef]
- Ali Khalifa, M.E.; Gouda, Z.A. The Antioxidative and Antiapoptotic Effects of Chlorophyllin on a Female Rat Spleen Exposed to Localized Gastric Radiotherapy. J. Biochem. Cell Biol. 2018, 1, 102. [Google Scholar]
- Nowacka, M.M.; Obuchowicz, E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: A new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides 2012, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.D.; Duman, R.S. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav. Pharm. 2007, 18, 391–418. [Google Scholar] [CrossRef] [PubMed]
- Nowacka-Chmielewska, M.M.; Paul-Samojedny, M.; Bielecka-Wajdman, A.M.; Barski, J.J.; Obuchowicz, E. Alterations in VEGF expression induced by antidepressant drugs in female rats under chronic social stress. Exp. Ther. Med. 2017, 13, 723–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, J.; Mitchell, B.; Appadurai, D.A.; Shakya, A.; Pierce, L.J.; Wang, H.; Spangrude, G.J. Vitamin C promotes maturation of T-cells. Antioxid. Redox Signal. 2013, 19, 2054–2067. [Google Scholar] [CrossRef] [PubMed]
- Heiser, P.; Sommer, O.; Schmidt, A.J.; Clement, H.W.; Hoinkes, A.; Hopt, U.T.; Schulz, E.; Krieg, J.C.; Dobschütz, E. Effects of antipsychotics and vitamin C on the formation of reactive oxygen species. J. Psychopharmacol. 2010, 24, 1499–1504. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Antioxidant and prooxidant properties of vitamin C. In Vitamin C in Health and Disease; Packer, L., Fuchs, J., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 59–73. [Google Scholar]
- Mandl, J.; Szarka, A.; Banhegyi, G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009, 157, 1097–1110. [Google Scholar] [CrossRef] [PubMed]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, S.; Salah, M. Differential Expression of CD3, TNF-α, and VEGF Induced by Olanzapine on the Spleen of Adult Male Albino Rats and the Possible Protective Role of Vitamin C. Biomedicines 2019, 7, 39. https://doi.org/10.3390/biomedicines7020039
Youssef S, Salah M. Differential Expression of CD3, TNF-α, and VEGF Induced by Olanzapine on the Spleen of Adult Male Albino Rats and the Possible Protective Role of Vitamin C. Biomedicines. 2019; 7(2):39. https://doi.org/10.3390/biomedicines7020039
Chicago/Turabian StyleYoussef, Sahar, and Marwa Salah. 2019. "Differential Expression of CD3, TNF-α, and VEGF Induced by Olanzapine on the Spleen of Adult Male Albino Rats and the Possible Protective Role of Vitamin C" Biomedicines 7, no. 2: 39. https://doi.org/10.3390/biomedicines7020039
APA StyleYoussef, S., & Salah, M. (2019). Differential Expression of CD3, TNF-α, and VEGF Induced by Olanzapine on the Spleen of Adult Male Albino Rats and the Possible Protective Role of Vitamin C. Biomedicines, 7(2), 39. https://doi.org/10.3390/biomedicines7020039