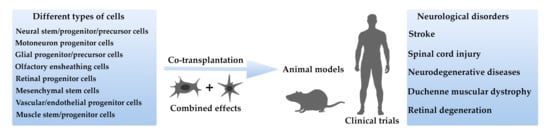
Combined Cell Therapy in the Treatment of Neurological Disorders
Abstract
:1. Introduction
2. Ischemic Stroke
2.1. Results of Animal Studies
2.2. Results of Clinical Trials
3. Spinal Cord Injury
3.1. Results of Animal Studies
3.2. Results of Clinical Trials
4. Neurodegenerative Diseases
Results of Animal Studies
5. Duchenne Muscular Dystrophy
Results of Clinical Trials
6. Retinal Degenerative Diseases
Results of Animal Studies
7. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Song, C.G.; Zhang, Y.Z.; Wu, H.N.; Cao, X.L.; Guo, C.J.; Li, Y.Q.; Zheng, M.H.; Han, H. Stem cells: A promising candidate to treat neurological disorders. Neural Regen. Res. 2018, 13, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Gögel, S.; Gubernator, M.; Minger, S.L. Progress and prospects: Stem cells and neurological diseases. Gene Ther. 2011, 18, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.; Zarriello, S.; Coats, A.; Nelson, C.; Kingsbury, C.; Gorsky, A.; Rajani, M.; Neal, E.G.; Borlongan, C.V. Stem cell therapy for neurological disorders: A focus on aging. Neurobiol. Dis. 2019, 126, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.W.; Kinder, H.A.; West, F.D. Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 2019, 9, e01214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokaia, Z.; Darsalia, V. Human Neural Stem Cells for Ischemic Stroke Treatment. In Results and Problems in Cell Differentiation; Springer: Cham, Switzerland, 2018; Volume 66, pp. 249–263. [Google Scholar]
- Liao, L.-Y.; Lau, B.-M.; Sánchez-Vidaña, D.; Gao, Q. Exogenous neural stem cell transplantation for cerebral ischemia. Neural Regen. Res. 2019, 14, 1129. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Puerta, G.J.; Marchal, J.A.; López-Ruiz, E.; Gálvez-Martín, P. Role of Mesenchymal Stromal Cells as Therapeutic Agents: Potential Mechanisms of Action and Implications in Their Clinical Use. J. Clin. Med. 2020, 9, 445. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Mishra, V.K.; Shih, H.-H.; Parveen, F.; Lenzen, D.; Ito, E.; Chan, T.-F.; Ke, L.-Y. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells 2020, 9, 1145. [Google Scholar] [CrossRef]
- Liao, S.; Luo, C.; Cao, B.; Hu, H.; Wang, S.; Yue, H.; Chen, L.; Zhou, Z. Endothelial Progenitor Cells for Ischemic Stroke: Update on Basic Research and Application. Stem Cells Int. 2017, 2017, 2193432. [Google Scholar] [CrossRef] [Green Version]
- Boese, A.C.; Le, Q.S.E.; Pham, D.; Hamblin, M.H.; Lee, J.P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res. Ther. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, Y.; Shen, T.; Gao, C. Induced migration of endothelial cells into 3D scaffolds by chemoattractants secreted by pro-inflammatory macrophages in situ. Regen. Biomater. 2017, 4, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Thayabaranathan, T.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; Owolabi, M.; Pandian, J.; et al. Global Stroke Statistics 2019. Int. J. Stroke 2020, 15, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, E344–E418. [Google Scholar] [PubMed]
- Ballester, B.R.; Maier, M.; Duff, A.; Cameirão, M.; Bermúdez, S.; Duarte, E.; Cuxart, A.; Rodríguez, S.; San Segundo Mozo, R.M.; Verschure, P.F.M.J. A critical time window for recovery extends beyond one-year post-stroke. J. Neurophysiol. 2019, 122, 350–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef]
- Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. [Google Scholar] [CrossRef]
- Singh, M.; Pandey, P.K.; Bhasin, A.; Padma, M.V.; Mohanty, S. Application of Stem Cells in Stroke: A Multifactorial Approach. Front. Neurosci. 2020, 14, 473. [Google Scholar] [CrossRef]
- Suda, S.; Nito, C.; Yokobori, S.; Sakamoto, Y.; Nakajima, M.; Sowa, K.; Obinata, H.; Sasaki, K.; Savitz, S.I.; Kimura, K. Recent Advances in Cell-Based Therapies for Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 6718. [Google Scholar] [CrossRef]
- Kumar, G.; Mukherjee, S.; Paliwal, P.; Tripathi, A.K.; Krishnamurthy, S.; Patnaik, R. Stem Cell-Based Therapy for Ischemic Stroke. In Advancement in the Pathophysiology of Cerebral Stroke; Springer: Singapore, 2019; pp. 103–121. [Google Scholar]
- Li, J.; Tang, Y.; Wang, Y.; Tang, R.; Jiang, W.; Yang, G.Y.; Gao, W.Q. Neurovascular recovery via cotransplanted neural and vascular progenitors leads to improved functional restoration after ischemic stroke in rats. Stem Cell Reports 2014, 3, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Nakagomi, N.; Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Saino, O.; Takata, M.; Yoshikawa, H.; Stern, D.M.; Matsuyama, T.; Taguchi, A. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells 2009, 27, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Farahmandnia, M.; Razi, Z.; Delavari, S.; Shakibajahromi, B.; Sarvestani, F.S.; Kazemi, S.; Semsar, M. Combination cell therapy with mesenchymal stem cells and neural stem cells for brain stroke in rats. Int. J. Stem Cells 2015, 8, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhou, Z.; Ju, X.; Zhou, Y.; Lan, J.; Chen, D.; Chen, H.; Liu, M.; Pang, L. Combined transplantation of mesenchymal stem cells and endothelial progenitor cells for tissue engineering: A systematic review and meta-analysis. Stem Cell Res. Ther. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, L.-Y.; Huang, F.-J.; Zhao, M.; Xie, J.-H.; Shi, J.; Wang, J.; Lin, X.-Z.; Zuo, H.; Wang, Y.-L.; Geng, T.-C. A Two-Year Follow-Up Study of Cotransplantation with Neural Stem/Progenitor Cells and Mesenchymal Stromal Cells in Ischemic Stroke Patients. Cell Transplant. 2014, 23, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Badhiwala, J.H.; Wilson, J.R.; Fehlings, M.G. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019, 18, 24–25. [Google Scholar] [CrossRef] [Green Version]
- Spinal Cord Injury. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 15 November 2020).
- Fehlings, M.G.; Tetreault, L.A.; Wilson, J.R.; Kwon, B.K.; Burns, A.S.; Martin, A.R.; Hawryluk, G.; Harrop, J.S. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Glob. Spine J. 2017, 7, 84S–94S. [Google Scholar] [CrossRef]
- Huang, H.; Young, W.; Skaper, S.; Chen, L.; Moviglia, G.; Saberi, H.; Al-Zoubi, Z.; Sharma, H.S.; Muresanu, D.; Sharma, A.; et al. Clinical Neurorestorative Therapeutic Guidelines for Spinal Cord Injury (IANR/CANR version 2019). J. Orthop. Transl. 2020, 20, 14–24. [Google Scholar] [CrossRef]
- Jin, M.C.; Medress, Z.A.; Azad, T.D.; Doulames, V.M.; Veeravagu, A. Stem cell therapies for acute spinal cord injury in humans: A review. Neurosurg. Focus 2019, 46, E10. [Google Scholar] [CrossRef]
- Vismara, I.; Papa, S.; Rossi, F.; Forloni, G.; Veglianese, P. Current Options for Cell Therapy in Spinal Cord Injury. Trends Mol. Med. 2017, 23, 831–849. [Google Scholar] [CrossRef]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal stem cells for spinal cord injury: Current options limitations, and future of cell therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef] [Green Version]
- Dasari, V.R. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J. Stem Cells 2014, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Baklaushev, V.P.; Bogush, V.G.; Kalsin, V.A.; Sovetnikov, N.N.; Samoilova, E.M.; Revkova, V.A.; Sidoruk, K.V.; Konoplyannikov, M.A.; Timashev, P.S.; Kotova, S.L.; et al. Tissue Engineered Neural Constructs Composed of Neural Precursor Cells, Recombinant Spidroin and PRP for Neural Tissue Regeneration. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanyuk, N.; Amemori, T.; Turnovcova, K.; Prochazka, P.; Onteniente, B.; Sykova, E.; Jendelova, P. Beneficial effect of human induced pluripotent stem cell-derived neural precursors in spinal cord injury repair. Cell Transplant. 2015, 24, 1781–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erceg, S.; Ronaghi, M.; Oria, M.; Roselló, M.G.; Aragó, M.A.P.; Lopez, M.G.; Radojevic, I.; Moreno-Manzano, V.; Rodríguez-Jiménez, F.J.; Bhattacharya, S.S.; et al. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells 2010, 28, 1541–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, M.; Pasbakhsh, P.; Soleimani, M.; Abbasi, M.; Hasanzadeh, G.; Modaresi, M.H.; Sobhani, A. Repair of spinal cord injury by co-transplantation of embryonic stem cell-derived motor neuron and olfactory ensheathing cell. Iran. Biomed. J. 2009, 13, 125–135. [Google Scholar]
- Park, D.Y.; Mayle, R.E.; Smith, R.L.; Corcoran-Schwartz, I.; Kharazi, A.I.; Cheng, I. Combined Transplantation of Human Neuronal and Mesenchymal Stem Cells following Spinal Cord Injury. Glob. Spine J. 2013, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Huang, H.; Xi, H.; Zhang, F.; Liu, Y.; Chen, D.; Xiao, J. A Prospective Randomized Double-Blind Clinical Trial Using a Combination of Olfactory Ensheathing Cells and Schwann Cells for the Treatment of Chronic Complete Spinal Cord Injuries. Cell Transpl. 2014, 23, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Ichim, T.E.; Solano, F.; Lara, F.; Paris, E.; Ugalde, F.; Rodriguez, J.P.; Minev, B.; Bogin, V.; Ramos, F.; Woods, E.J.; et al. Feasibility of combination allogeneic stem cell therapy for spinal cord injury: A case report. Int. Arch. Med. 2010, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Moviglia, G.A.; Varela, G.; Brizuela, J.A.; Moviglia Brandolino, M.T.; Farina, P.; Etchegaray, G.; Piccone, S.; Hirsch, J.; Martinez, G.; Marino, S.; et al. Case report on the clinical results of a combined cellular therapy for chronic spinal cord injured patients. Spinal Cord 2009, 47, 499–503. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Cova, L.; Armentero, M.-T. 1980–2011: Parkinson’s Disease and Advance in Stem Cell Research. In Towards New Therapies for Parkinson’s Disease; InTech: London, UK, 2011. [Google Scholar]
- Bratt-Leal, A.M.; Loring, J.F. Stem cells for parkinson’s disease. In Translational Neuroscience: Fundamental Approaches for Neurological Disorders; Springer: Cham, Switzerland, 2016; pp. 187–201. ISBN 9781489976543. [Google Scholar]
- Chou, C.H.; Fan, H.C.; Hueng, D.Y. Potential of Neural Stem Cell-Based Therapy for Parkinson’s Disease. Parkinsons. Dis. 2015, 2015, 571475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, N.; Seth, K.; Khanna, V.K.; Ansari, R.W.; Agrawal, A.K. Long-term functional restoration by neural progenitor cell transplantation in rat model of cognitive dysfunction: Co-transplantation with olfactory ensheathing cells for neurotrophic factor support. Int. J. Dev. Neurosci. 2009, 27, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zheng, X.Y.; Zhang, H.L.; Luo, Q. Kainic acid-induced neurodegenerative model: Potentials and limitations. J. Biomed. Biotechnol. 2011, 2011, 457079. [Google Scholar]
- Agrawal, A.K.; Shukla, S.; Chaturvedi, R.K.; Seth, K.; Srivastava, N.; Ahmad, A.; Seth, P.K. Olfactory ensheathing cell transplantation restores functional deficits in rat model of Parkinson’s disease: A cotransplantation approach with fetal ventral mesencephalic cells. Neurobiol. Dis. 2004, 16, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Muscular Dystrophy: Hope Through Research|National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Muscular-Dystrophy-Hope-Through-Research (accessed on 15 November 2020).
- Cyrulnik, S.E.; Fee, R.J.; De Vivo, D.C.; Goldstein, E.; Hinton, V.J. Delayed Developmental Language Milestones in Children with Duchenne’s Muscular Dystrophy. J. Pediatr. 2007, 150, 474–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Messina, S.; Trifirò, G. Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Ryder, S.; Leadley, R.M.; Armstrong, N.; Westwood, M.; De Kock, S.; Butt, T.; Jain, M.; Kleijnen, J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: An evidence review. Orphanet J. Rare Dis. 2017, 12, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aartsma-Rus, A.; Ginjaar, I.B.; Bushby, K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J. Med. Genet. 2016, 53, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, D.; Okurowska Zawada, B.; Paszko Patej, G.; Kawnik, K.; Kulak, W. Duchenne muscular dystrophy: Current cell therapies. Ther. Adv. Neurol. Disord. 2015, 8, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef] [Green Version]
- Mah, J.K. Current and Emerging Treatment Strategies for Duchenne Muscular Dystrophy; Dove Medical Press Ltd.: London, UK, 2016; pp. 1795–1807. [Google Scholar]
- Mendell, J.R.; Kissel, J.T.; Amato, A.A.; King, W.; Signore, L.; Prior, T.W.; Sahenk, Z.; Benson, S.; McAndrew, P.E.; Rice, R.; et al. Myoblast Transfer in the Treatment of Duchenne’s Muscular Dystrophy. N. Engl. J. Med. 1995, 333, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Mouly, V.; Aamiri, A.; Périé, S.; Mamchaoui, K.; Barani, A.; Bigot, A.; Bouazza, B.; François, V.; Furling, D.; Jacquemin, V.; et al. Myoblast transfer therapy: Is there any light at the end of the tunnel? Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2005, 24, 128–133. [Google Scholar]
- Skuk, D.; Goulet, M.; Roy, B.; Chapdelaine, P.; Bouchard, J.-P.; Roy, R.; Dugré, F.J.; Sylvain, M.; Lachance, J.-G.; Deschênes, L.; et al. Dystrophin Expression in Muscles of Duchenne Muscular Dystrophy Patients After High-Density Injections of Normal Myogenic Cells. J. Neuropathol. Exp. Neurol. 2006, 65, 371–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skuk, D.; Goulet, M.; Roy, B.; Piette, V.; Côté, C.H.; Chapdelaine, P.; Hogrel, J.Y.; Paradis, M.; Bouchard, J.P.; Sylvain, M.; et al. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: Eighteen months follow-up. Neuromuscul. Disord. 2007, 17, 38–46. [Google Scholar] [CrossRef]
- Danisovic, L.; Culenova, M.; Csobonyeiova, M. Induced Pluripotent Stem Cells for Duchenne Muscular Dystrophy Modeling and Therapy. Cells 2018, 7, 253. [Google Scholar] [CrossRef] [Green Version]
- Briggs, D.; Morgan, J.E. Recent progress in satellite cell/myoblast engraftment—Relevance for therapy. FEBS J. 2013, 280, 4281–4293. [Google Scholar] [CrossRef] [Green Version]
- Chang, N.C.; Chevalier, F.P.; Rudnicki, M.A. Satellite Cells in Muscular Dystrophy—Lost in Polarity. Trends Mol. Med. 2016, 22, 479–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossu, G.; Previtali, S.C.; Napolitano, S.; Cicalese, M.P.; Tedesco, F.S.; Nicastro, F.; Noviello, M.; Roostalu, U.; Natali Sora, M.G.; Scarlato, M.; et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol. Med. 2015, 7, 1513–1528. [Google Scholar] [CrossRef]
- Ichim, T.E.; Alexandrescu, D.T.; Solano, F.; Lara, F.; Campion, R.D.N.; Paris, E.; Woods, E.J.; Murphy, M.P.; Dasanu, C.A.; Patel, A.N.; et al. Mesenchymal stem cells as anti-inflammatories: Implications for treatment of Duchenne muscular dystrophy. Cell Immunol. 2010, 260, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Berenstein, P.; Kronfeld, N.; Morgoulis, D.; Ziv-Av, A.; Goldstein, H.; Kazimirsky, G.; Cazacu, S.; Meir, R.; Popovtzer, R.; et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials 2018, 174, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Elhussieny, A.; Nogami, K.; Sakai-Takemura, F.; Maruyama, Y.; Omar Abdelbakey, A.; Abou El-kheir, W.; Takeda, S.; Miyagoe-Suzuki, Y. Mesenchymal Stem Cells for Regenerative Medicine for Duchenne Muscular Dystrophy. In Muscular Dystrophy—Advances in Cellular and Molecular Basis, Diagnosis and Therapeutic Strategies [Working Title]; IntechOpen: London, UK, 2020. [Google Scholar]
- Kazemi, Y.; Nikmanesh, Z.; Khosravi, M.; Hassanzadeh, Z. The relationship of self-esteem and attributional styles with self-handicapping in primary schools. Int. J. Sch. Heal. 2020, 5, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Klimczak, A.; Zimna, A.; Malcher, A.; Kozlowska, U.; Futoma, K.; Czarnota, J.; Kemnitz, P.; Bryl, A.; Kurpisz, M. Co-Transplantation of Bone Marrow-MSCs and Myogenic Stem/Progenitor Cells from Adult Donors Improves Muscle Function of Patients with Duchenne Muscular Dystrophy. Cells 2020, 9, 1119. [Google Scholar] [CrossRef] [PubMed]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.-S.; McNamara, J.O.; Williams, S.M. The Retina; Chicago Press: Chicago, IL, USA, 2001. [Google Scholar]
- Lanza, R.; Langer, R.; Vacanti, J.P. Principles of Tissue Engineering: Fourth Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780123983589. [Google Scholar]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Ammar, M.J.; Hsu, J.; Chiang, A.; Ho, A.C.; Regillo, C.D. Age-related macular degeneration therapy. Curr. Opin. Ophthalmol. 2020, 31, 215–221. [Google Scholar] [CrossRef]
- Aziz, K.; Zarbin, M.A.; Singh, M.S. Clinical Trials of Retinal Cell Therapy; Humana Press: Cham, Switzerland, 2019; pp. 245–265. [Google Scholar]
- Li, Z.; Zeng, Y.; Chen, X.; Li, Q.; Wu, W.; Xue, L.; Xu, H.; Yin, Z.Q. Neural stem cells transplanted to the subretinal space of rd1 mice delay retinal degeneration by suppressing microglia activation. Cytotherapy 2016, 18, 771–784. [Google Scholar] [CrossRef] [PubMed]
- McGill, T.J.; Cottam, B.; Lu, B.; Wang, S.; Girman, S.; Tian, C.; Huhn, S.L.; Lund, R.D.; Capela, A. Transplantation of human central nervous system stem cells—Neuroprotection in retinal degeneration. Eur. J. Neurosci. 2012, 35, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Kumar, S.; Mok, P. Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int. J. Mol. Sci. 2017, 18, 1406. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.J.; Li, S.Y.; Qu, L.H.; Meng, X.H.; Wang, Y.; Xu, H.W.; Liang, Z.Q.; Yin, Z.Q. Long-Term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Res. Ther. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhai, W.; Gao, L.; Qu, L.; Li, Y.; Zeng, Y.; Li, Q.; Xu, H.; Yin, Z.Q. Combined Transplantation of Olfactory Ensheathing Cells with Rat Neural Stem Cells Enhanced the Therapeutic Effect in the Retina of RCS Rats. Front. Cell. Neurosci. 2020, 14, 52. [Google Scholar] [CrossRef]
- Qu, L.; Gao, L.; Xu, H.; Duan, P.; Zeng, Y.; Liu, Y.; Yin, Z.Q. Combined transplantation of human mesenchymal stem cells and human retinal progenitor cells into the subretinal space of RCS rats. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Ford, M.C.; Lavik, E.B.; Madri, J.A. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: An in vitro study. J. Neurosci. Res. 2006, 84, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tan, Z.; Huang, X.; Cheng, X.; Yuan, Y.; Qin, S.; Wang, D.; Hu, X.; Gu, Y.; Qian, W.-J.; et al. Direct neuronal reprogramming of olfactory ensheathing cells for CNS repair. Cell Death Dis. 2019, 10, 646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; He, C. Olfactory ensheathing cells: Biology in neural development and regeneration. Prog. Neurobiol. 2010, 92, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Falkner, S.; Grade, S.; Dimou, L.; Conzelmann, K.-K.; Bonhoeffer, T.; Götz, M.; Hübener, M. Transplanted embryonic neurons integrate into adult neocortical circuits. Nature 2016, 539, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Ottoboni, L.; von Wunster, B.; Martino, G. Therapeutic Plasticity of Neural Stem Cells. Front. Neurol. 2020, 11, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Liu, F.; Zhou, Y.; Jin, B.; Sun, Q.; Guo, S. Immunosuppressive Property of MSCs Mediated by Cell Surface Receptors. Front. Immunol. 2020, 11, 1076. [Google Scholar] [CrossRef]
- Yarygin, K.N.; Lupatov, A.Y.; Sukhikh, G.T. Modulation of immune responses by mesenchymal stromal cells. Bull. Exp. Biol. Med. 2016, 161, 561–565. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [Green Version]
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria know no boundaries: Mechanisms and functions of intercellular mitochondrial transfer. Front. Cell Dev. Biol. 2016, 4, 107. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Cselenyák, A.; Pankotai, E.; Horváth, E.M.; Kiss, L.; Lacza, Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 2010, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, M.C.B.; McDonald, C.A.; Allison, B.J.; Fahey, M.C.; Jenkin, G.; Miller, S.L. Perinatal brain injury as a consequence of preterm birth and intrauterine inflammation: Designing targeted stem cell therapies. Front. Neurosci. 2017, 11, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Pathology | Species | Cell Types | Way of Delivery | Immunosuppression | Time of Delivery | Advantages of Combined over Single Cell Therapy |
|---|---|---|---|---|---|---|---|
| Li et al. [22] | MCAO stroke model | Rats | Fetal murine NPC + VPC | IC sequential injections of both types of cells; | Cyclosporine A | 24 h after MCAO | Functional recovery; stroke volume reduction; neural and vascular graft better survival; stimulated migration, differentiation, and maturation of NPC |
| Nakagomi et al. [23] | MCAO stroke model | Mice | Murine adult NSC + EC | IC sequential injections of both types of cells | The recipients were immunosuppressive mice | 7 days after MCAO | Improved cortical function; enhanced survival, proliferation and differentiation of NSC |
| Hosseini et al. [24] | MCAO stroke model | Rats | Rat bone marrow MSC + fetal NSC | IVT | No | MSC 1 day after and NSC 7 days after MCAO | Better functional recovery; reduction of stroke volume |
| Erceg [37] | SCI complete transection model | Rats | Human ESC-derived OPC + MPC | IS | No | Immediately after SCI | Electrophysiological improvement |
| Salehi et al. [38] | SCI contusion model | Rats | Murine ESMN + OEC | IS | Cyclosporine A | 9 days after SCI | Functional recovery, but no significant difference compared to single cell type therapy; enhanced ESMN survival; better tissue sparing and myelin ratio |
| Park et al. [39] | SCI contusion model | Rats | Human bone marrow MSC + fetal NSC | IV + IS | No | MSC IV immediately after and NSC IS 7 days after SCI | Functional recovery, but no significant difference compared to single cell type therapy |
| Srivastava et al. [47] | Kainic acid-induced model of Alzheimer’s disease | Rats | Rat adult OEC+ fetal NPC | IC | No | 4 weeks post-lesioning | Learning and memory recovery; enhanced expression of choline acetyltransferase and cholinergic receptors binding |
| Agrawal et al. [49] | 6-hydroxydopamine—lesioned model of Parkinson’s disease | Rats | Rat adult OEC + fetal VMC | IC | No | 5 weeks post-lesioning | Functional recovery; more tyrosine hydroxylase-positive cells and higher density of tyrosine hydroxylase-positive fibers |
| Zhai et al. [80] | Model of inherited retinal degeneration (Royal College of Surgeons (RCS) rats) | Rats | Rat adult OEC + fetal NSC | Into subretinal space | The recipients were RCS rats | 4 weeks postnatally | Electrophysiological improvement according to electroretinogram; activation of endogenous retinal stem cells; reduced gliosis; enhanced NSC migration; better photoreceptor survival |
| Qu et al. [81] | Model of inherited retinal degeneration (Royal College of Surgeons (RCS) rats) | Rats | Human HRPC + human bone marrow MSC | Into subretinal space | The recipients were RCS rats | 3 weeks postnatally | Electrophysiological improvement according to electroretinogram; better survival, migration, and differentiation of HRPC; reduced gliosis |
| Study | Pathology | Phases of Clinical Research | Cell Types | Way of Delivery | Time of Delivery | Advantages of Combined over Single Cell Therapy |
|---|---|---|---|---|---|---|
| Qiao et al. [26] | Ischemic stroke | Phase I/II eight patients no control group/placebo control; no randomization; 2-year follow-up | Human fetal NSPC + umbilical cord MSC | MSC IV multiple times + NSPC through the cerebellomedullary cistern one time | Acute phase post-stroke | Better NIHSS, BI, mRS scores, but no control group |
| Klimczak et al. [70] | Dushenne muscular dystrophy | Phase I/II three patients no control group/placebo control; no randomization; 6-month follow-up | Human allogenic bone marrow MSC + SM-SPC | Direct IM | Patients between 11 and 22 years old | Electrophysiological improvement according to ENMG; decreased blood creatine kinase level; normalized profile of proinflammatory cytokines; decrease in the content of adipose and fibrous tissue in muscles; increased level of dystrophin expression |
| Moviglia et al. [42] | SCI | Case reports eight patients | Human autologous bone marrow MSC + spinal cord specific effector T cells + autologous NSC | MSC IA + 18 days later effector T cells IV + NSC IA | Chronic phase post injury | Increased ASIA score grade, but no control group |
| Ichim et al. [41] | SCI | Case report one patient | Human allogenic umbilical cord blood CD34 + placental derived MSC | IT | Within first year after injury | Functional improvement, but no control group |
| Chen et al. [40] | SCI | Phase I/II 28 patients prospective randomized double-blind no placebo 12-month follow-up | Human fetal OEC + SC | IS | At least 12 months after injury | Functional recovery, but no significant difference compared to single cell type therapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namestnikova, D.D.; Cherkashova, E.A.; Sukhinich, K.K.; Gubskiy, I.L.; Leonov, G.E.; Gubsky, L.V.; Majouga, A.G.; Yarygin, K.N. Combined Cell Therapy in the Treatment of Neurological Disorders. Biomedicines 2020, 8, 613. https://doi.org/10.3390/biomedicines8120613
Namestnikova DD, Cherkashova EA, Sukhinich KK, Gubskiy IL, Leonov GE, Gubsky LV, Majouga AG, Yarygin KN. Combined Cell Therapy in the Treatment of Neurological Disorders. Biomedicines. 2020; 8(12):613. https://doi.org/10.3390/biomedicines8120613
Chicago/Turabian StyleNamestnikova, Daria D., Elvira A. Cherkashova, Kirill K. Sukhinich, Ilya L. Gubskiy, Georgy E. Leonov, Leonid V. Gubsky, Alexander G. Majouga, and Konstantin N. Yarygin. 2020. "Combined Cell Therapy in the Treatment of Neurological Disorders" Biomedicines 8, no. 12: 613. https://doi.org/10.3390/biomedicines8120613
APA StyleNamestnikova, D. D., Cherkashova, E. A., Sukhinich, K. K., Gubskiy, I. L., Leonov, G. E., Gubsky, L. V., Majouga, A. G., & Yarygin, K. N. (2020). Combined Cell Therapy in the Treatment of Neurological Disorders. Biomedicines, 8(12), 613. https://doi.org/10.3390/biomedicines8120613