Breath-Taking Perspectives and Preliminary Data toward Early Detection of Chronic Liver Diseases
Abstract
:1. Introduction
2. Breath Analysis Is an Attractive Means for Chronic Liver Diseases Early Detection
- (1)
- Breath analysis could be used for chronic liver disease detection.
- (2)
- Overall, a wide spectrum of chronic liver diseases and etiologies were explored.
- (3)
- (4)
- Small sample size and lack of validation demote these compounds to the proof of principle stage.
- (5)
- Additional studies with reliable power and implementing strategies to control the exposure confounder must be performed to transition these potential biomarkers from proof of principle to marketed breath tests.
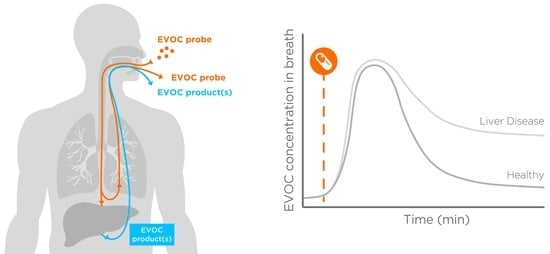
3. Transitioning from Endogenous Biomarkers to Exogenous Reporters
4. Advantages of GRAS Compounds over 13C-Reporters Breath Tests, and Blood Tests
- (1)
- Assessing multiple metabolic routes at once, by administering combination of compounds metabolized by different pathways.
- (2)
- (3)
- Increase technical sensitivity by administrating doses that provide a signal several folds over background levels.
5. Experimental Section: Preliminary Results from Healthy Subjects Support the Feasibility of the EVOC Probe Approach
- Develop analytical capability for breath detection of these compounds.
- Identify compounds that appear on breath after oral administration at the chosen doses.
- Estimate time points and a time range for breath collection to observe a spike followed by a washout.
- (1)
- All the compounds showed baseline breath levels close or similar to ambient after an overnight fasting, suggesting that implementation of this procedure, reduces the confounder represented by random dietary exposure.
- (2)
- Oral administration of the compounds via a liquid formulation induced a spike on breath in all the subjects and in all the experiments, overcoming unreliable appearance on breath we experienced using capsules (data not shown), providing the benefit of detecting the compounds more easily, and avoiding the confounder represented by ambient contamination.
- (3)
- The reduction observed after 40–60 min suggest that the absorption phase is mainly completed within the first hour.
6. Discussion
7. Materials and Methods
7.1. Participants and Protocol
7.2. Breath Biopsy Collection
7.3. Analytical Methodology
8. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Holzhutter, H.G.; Wuensch, T.; Gajowski, R.; Berndt, N.; Bulik, S.; Meierhofer, D.; Stockmann, M. A novel variant of the (13)c-methacetin liver function breath test that eliminates the confounding effect of individual differences in systemic co2 kinetics. Arch. Toxicol. 2020, 94, 401–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD-Cirrhosis-Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, P.; Younossi, Z.M. The global burden of cirrhosis: A review of disability-adjusted life-years lost and unmet needs. J. Hepatol. 2021, 75 (Suppl. 1), S3–S13. [Google Scholar] [CrossRef]
- Gandhi, C.R. Hepatic stellate cell activation and pro-fibrogenic signals. J. Hepatol. 2017, 67, 1104–1105. [Google Scholar] [CrossRef] [Green Version]
- Pinzani, M.; Romanelli, R.G.; Magli, S. Progression of fibrosis in chronic liver diseases: Time to tally the score. J. Hepatol. 2001, 34, 764–767. [Google Scholar] [CrossRef]
- Puche, J.E.; Saiman, Y.; Friedman, S.L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 2013, 3, 1473–1492. [Google Scholar]
- Sharma, A.; Nagalli, S. Chronic liver disease. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Harman, D.J.; Ryder, S.D.; James, M.W.; Jelpke, M.; Ottey, D.S.; Wilkes, E.A.; Card, T.R.; Aithal, G.P.; Guha, I.N. Direct targeting of risk factors significantly increases the detection of liver cirrhosis in primary care: A cross-sectional diagnostic study utilising transient elastography. BMJ Open 2015, 5, e007516. [Google Scholar] [CrossRef] [Green Version]
- Saleh, H.A.; Abu-Rashed, A.H. Liver biopsy remains the gold standard for evaluation of chronic hepatitis and fibrosis. J. Gastrointestin. Liver Dis. 2007, 16, 425–426. [Google Scholar] [PubMed]
- Davison, B.A.; Harrison, S.A.; Cotter, G.; Alkhouri, N.; Sanyal, A.; Edwards, C.; Colca, J.R.; Iwashita, J.; Koch, G.G.; Dittrich, H.C. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J. Hepatol. 2020, 73, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the british society of gastroenterology, the royal college of radiologists and the royal college of pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef]
- Nallagangula, K.S.; Nagaraj, S.K.; Venkataswamy, L.; Chandrappa, M. Liver fibrosis: A compilation on the biomarkers status and their significance during disease progression. Future Sci. OA 2018, 4, FSO250. [Google Scholar] [CrossRef] [Green Version]
- Soresi, M.; Giannitrapani, L.; Cervello, M.; Licata, A.; Montalto, G. Non invasive tools for the diagnosis of liver cirrhosis. World J. Gastroenterol. 2014, 20, 18131–18150. [Google Scholar] [CrossRef] [Green Version]
- Castera, L.; Foucher, J.; Bernard, P.H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; de Ledinghen, V. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatology 2010, 51, 828–835. [Google Scholar] [CrossRef] [PubMed]
- McDonald, N.; Eddowes, P.J.; Hodson, J.; Semple, S.I.K.; Davies, N.P.; Kelly, C.J.; Kin, S.; Phillips, M.; Herlihy, A.H.; Kendall, T.J.; et al. Multiparametric magnetic resonance imaging for quantitation of liver disease: A two-centre cross-sectional observational study. Sci. Rep. 2018, 8, 9189. [Google Scholar] [CrossRef] [PubMed]
- Wilder, J.; Patel, K. The clinical utility of fibroscan((r)) as a noninvasive diagnostic test for liver disease. Med. Devices 2014, 7, 107–114. [Google Scholar]
- Eriksson, S.; Fraser, J.R.; Laurent, T.C.; Pertoft, H.; Smedsrod, B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp. Cell Res. 1983, 144, 223–228. [Google Scholar] [CrossRef]
- Gressner, A.M.; Haarmann, R. Hyaluronic acid synthesis and secretion by rat liver fat storing cells (perisinusoidal lipocytes) in culture. Biochem. Biophys. Res. Commun. 1988, 151, 222–229. [Google Scholar] [CrossRef]
- Guechot, J.; Laudat, A.; Loria, A.; Serfaty, L.; Poupon, R.; Giboudeau, J. Diagnostic accuracy of hyaluronan and type iii procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis c evaluated by roc curve analysis. Clin. Chem. 1996, 42, 558–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lydatakis, H.; Hager, I.P.; Kostadelou, E.; Mpousmpoulas, S.; Pappas, S.; Diamantis, I. Non-invasive markers to predict the liver fibrosis in non-alcoholic fatty liver disease. Liver Int. 2006, 26, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.J.; Mann, D.A.; Iredale, J.P. Tissue inhibitors of metalloproteinases, hepatic stellate cells and liver fibrosis. J. Gastroenterol. Hepatol. 1998, 13 (Suppl. 1), S33–S38. [Google Scholar] [CrossRef]
- Badra, G.; Lotfy, M.; El-Refaie, A.; Obada, M.; Abdelmonem, E.; Kandeel, S.; Fathy, A. Significance of serum matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in chronic hepatitis c patients. Acta Microbiol. Immunol. Hung. 2010, 57, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.E.; Alonso, D.F.; Yoshiji, H.; Thorgeirsson, U.P. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur. J. Cell Biol. 1997, 74, 111–122. [Google Scholar]
- Herbst, H.; Wege, T.; Milani, S.; Pellegrini, G.; Orzechowski, H.D.; Bechstein, W.O.; Neuhaus, P.; Gressner, A.M.; Schuppan, D. Tissue inhibitor of metalloproteinase-1 and -2 rna expression in rat and human liver fibrosis. Am. J. Pathol. 1997, 150, 1647–1659. [Google Scholar] [PubMed]
- Kossakowska, A.E.; Edwards, D.R.; Lee, S.S.; Urbanski, L.S.; Stabbler, A.L.; Zhang, C.L.; Phillips, B.W.; Zhang, Y.; Urbanski, S.J. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am. J. Pathol. 1998, 153, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Prakobwong, S.; Pinlaor, S.; Yongvanit, P.; Sithithaworn, P.; Pairojkul, C.; Hiraku, Y. Time profiles of the expression of metalloproteinases, tissue inhibitors of metalloproteases, cytokines and collagens in hamsters infected with opisthorchis viverrini with special reference to peribiliary fibrosis and liver injury. Int. J. Parasitol. 2009, 39, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.M.; Timms, P.; Campbell, S.; MacSween, R.N.; Morris, A.J. Plasma levels of matrix metalloproteinase-2 (mmp-2) and tissue inhibitors of metalloproteinases -1 and -2 (timp-1 and timp-2) as noninvasive markers of liver disease in chronic hepatitis c: Comparison using roc analysis. Dig. Dis. Sci. 1999, 44, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Collazos, J.; Diaz, F. Role of the measurement of serum procollagen type iii n-terminal peptide in the evaluation of liver diseases. Clin. Chim. Acta 1994, 227, 37–43. [Google Scholar] [CrossRef]
- Entzian, P.; Huckstadt, A.; Kreipe, H.; Barth, J. Determination of serum concentrations of type iii procollagen peptide in mechanically ventilated patients. Pronounced augmented concentrations in the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1990, 142, 1079–1082. [Google Scholar] [CrossRef]
- Horslev-Petersen, K. Circulating extracellular matrix components as markers for connective tissue response to inflammation. A clinical and experimental study with special emphasis on serum aminoterminal type iii procollagen peptide in rheumatic diseases. Dan. Med. Bull. 1990, 37, 308–329. [Google Scholar]
- Waydhas, C.; Nast-Kolb, D.; Trupka, A.; Lenk, S.; Duswald, K.H.; Schweiberer, L.; Jochum, M. Increased serum concentrations of procollagen type iii peptide in severely injured patients: An indicator of fibrosing activity? Crit. Care Med. 1993, 21, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Q.; Zhou, X.; Huang, P.; Wei, J.; Wang, W.; Zheng, S. The performance of enhanced liver fibrosis (elf) test for the staging of liver fibrosis: A meta-analysis. PLoS ONE 2014, 9, e92772. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with hiv/hcv coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Harrison, S.A.; Ratziu, V.; Boursier, J.; Francque, S.; Bedossa, P.; Majd, Z.; Cordonnier, G.; Sudrik, F.B.; Darteil, R.; Liebe, R.; et al. A blood-based biomarker panel (nis4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 970–985. [Google Scholar] [CrossRef]
- Gines, P.; Arroyo, V.; Quintero, E.; Planas, R.; Bory, F.; Cabrera, J.; Rimola, A.; Viver, J.; Camps, J.; Jimenez, W.; et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology 1987, 93, 234–241. [Google Scholar] [CrossRef]
- Salerno, F.; Badalamenti, S.; Incerti, P.; Tempini, S.; Restelli, B.; Bruno, S.; Bellati, G.; Roffi, L. Repeated paracentesis and i.V. Albumin infusion to treat ’tense’ ascites in cirrhotic patients. A safe alternative therapy. J. Hepatol. 1987, 5, 102–108. [Google Scholar] [CrossRef]
- Gaude, E.; Nakhleh, M.K.; Patassini, S.; Boschmans, J.; Allsworth, M.; Boyle, B.; van der Schee, M.P. Targeted breath analysis: Exogenous volatile organic compounds (evoc) as metabolic pathway-specific probes. J. Breath Res. 2019, 13, 032001. [Google Scholar] [CrossRef] [Green Version]
- Dweik, R.A.; Amann, A. Exhaled breath analysis: The new frontier in medical testing. J. Breath Res. 2008, 2, 030301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, T.C.; Tan, C.E.; Wang, S.Y.; Lin, O.A.; Su, B.H.; Hsu, M.T.; Lin, J.; Cheng, Y.Y.; Chen, C.S.; Yang, Y.C.; et al. Human breathomics database. Database 2020, 2020, baz139. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Vishinkin, R.; Barash, O.; Nakhleh, M.K.; Haick, H. Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem. Soc. Rev. 2018, 47, 4781–4859. [Google Scholar] [CrossRef]
- Stavropoulos, G.; van Munster, K.; Ferrandino, G.; Sauca, M.; Ponsioen, C.; van Schooten, F.J.; Smolinska, A. Liver impairment-the potential application of volatile organic compounds in hepatology. Metabolites 2021, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Tisch, U.; Schlesinger, I.; Ionescu, R.; Nassar, M.; Axelrod, N.; Robertman, D.; Tessler, Y.; Azar, F.; Marmur, A.; Aharon-Peretz, J.; et al. Detection of alzheimer’s and parkinson’s disease from exhaled breath using nanomaterial-based sensors. Nanomedicine 2013, 8, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Tiele, A.; Wicaksono, A.; Daulton, E.; Ifeachor, E.; Eyre, V.; Clarke, S.; Timings, L.; Pearson, S.; Covington, J.A.; Li, X. Breath-based non-invasive diagnosis of alzheimer’s disease: A pilot study. J. Breath Res. 2020, 14, 026003. [Google Scholar] [CrossRef]
- Phillips, M.; Sabas, M.; Greenberg, J. Increased pentane and carbon disulfide in the breath of patients with schizophrenia. J. Clin. Pathol. 1993, 46, 861–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, M.; Erickson, G.A.; Sabas, M.; Smith, J.P.; Greenberg, J. Volatile organic compounds in the breath of patients with schizophrenia. J. Clin. Pathol. 1995, 48, 466–469. [Google Scholar] [CrossRef] [Green Version]
- Broza, Y.Y.; Har-Shai, L.; Jeries, R.; Cancilla, J.C.; Glass-Marmor, L.; Lejbkowicz, I.; Torrecilla, J.S.; Yao, X.; Feng, X.; Narita, A.; et al. Exhaled breath markers for nonimaging and noninvasive measures for detection of multiple sclerosis. ACS Chem. Neurosci. 2017, 8, 2402–2413. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, R.; Broza, Y.; Shaltieli, H.; Sadeh, D.; Zilberman, Y.; Feng, X.; Glass-Marmor, L.; Lejbkowicz, I.; Mullen, K.; Miller, A.; et al. Detection of multiple sclerosis from exhaled breath using bilayers of polycyclic aromatic hydrocarbons and single-wall carbon nanotubes. ACS Chem. Neurosci. 2011, 2, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.; Cataneo, R.N.; Saunders, C.; Hope, P.; Schmitt, P.; Wai, J. Volatile biomarkers in the breath of women with breast cancer. J. Breath Res. 2010, 4, 026003. [Google Scholar] [CrossRef]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; de Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2013, 100, 144–150. [Google Scholar] [CrossRef]
- Woodfield, G.; Belluomo, I.; Lin, G.; Boshier, P.; Romano, A.; Martin, J.; Groves, C.; Marelli, L.; Saunders, B.; Atkin, W.; et al. Ptu-072 breath testing for colorectal polyps and cancer- a load of hot air? Gut 2018, 67, A206–A208. [Google Scholar]
- Ulanowska, A.; Kowalkowski, T.; Trawinska, E.; Buszewski, B. The application of statistical methods using vocs to identify patients with lung cancer. J. Breath Res. 2011, 5, 046008. [Google Scholar] [CrossRef]
- Filipiak, W.; Filipiak, A.; Sponring, A.; Schmid, T.; Zelger, B.; Ager, C.; Klodzinska, E.; Denz, H.; Pizzini, A.; Lucciarini, P.; et al. Comparative analyses of volatile organic compounds (vocs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. J. Breath Res. 2014, 8, 027111. [Google Scholar] [CrossRef]
- Rudnicka, J.; Walczak, M.; Kowalkowski, T.; Jezierski, T.; Buszewski, B. Determination of volatile organic compounds as potential markers of lung cancer by gas chromatography–mass spectrometry versus trained dogs. Sens. Actuators B Chem. 2014, 202, 615–621. [Google Scholar] [CrossRef]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation spme-gc/ms. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 2643–2651. [Google Scholar] [CrossRef]
- Wehinger, A.; Schmid, A.; Mechtcheriakov, S.; Ledochowski, M.; Grabmer, C.; Gastl, G.A.; Amann, A. Lung cancer detection by proton transfer reaction mass-spectrometric analysis of human breath gas. Int. J. Mass Spectrom. 2007, 265, 49–59. [Google Scholar] [CrossRef]
- Phillips, M.; Gleeson, K.; Hughes, J.M.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Einoch Amor, R.; Nakhleh, M.K.; Barash, O.; Haick, H. Breath analysis of cancer in the present and the future. Eur. Respir. Rev. 2019, 28, 190002. [Google Scholar] [CrossRef] [PubMed]
- Paredi, P.; Kharitonov, S.A.; Barnes, P.J. Elevation of exhaled ethane concentration in asthma. Am. J. Respir. Crit. Care Med. 2000, 162, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Olopade, C.O.; Zakkar, M.; Swedler, W.I.; Rubinstein, I. Exhaled pentane levels in acute asthma. Chest 1997, 111, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.A.; Yates, D.; Robbins, R.A.; Logan-Sinclair, R.; Shinebourne, E.A.; Barnes, P.J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994, 343, 133–135. [Google Scholar] [CrossRef]
- Horvath, I.; Donnelly, L.E.; Kiss, A.; Kharitonov, S.A.; Lim, S.; Chung, K.F.; Barnes, P.J. Combined use of exhaled hydrogen peroxide and nitric oxide in monitoring asthma. Am. J. Respir. Crit. Care Med. 1998, 158, 1042–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallinga, J.W.; Robroeks, C.M.; van Berkel, J.J.; Moonen, E.J.; Godschalk, R.W.; Jobsis, Q.; Dompeling, E.; Wouters, E.F.; van Schooten, F.J. Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin. Exp. Allergy 2010, 40, 68–76. [Google Scholar] [PubMed]
- Corradi, M.; Rubinstein, I.; Andreoli, R.; Manini, P.; Caglieri, A.; Poli, D.; Alinovi, R.; Mutti, A. Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, J.J.; Dallinga, J.W.; Moller, G.M.; Godschalk, R.W.; Moonen, E.J.; Wouters, E.F.; Van Schooten, F.J. A profile of volatile organic compounds in breath discriminates copd patients from controls. Respir. Med. 2010, 104, 557–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekhuijzen, P.N.; Aben, K.K.; Dekker, I.; Aarts, L.P.; Wielders, P.L.; van Herwaarden, C.L.; Bast, A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996, 154, 813–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Reilly, P.; Bailey, W. Clinical use of exhaled biomarkers in copd. Int. J. Chron. Obstruct. Pulmon. Dis. 2007, 2, 403–408. [Google Scholar] [PubMed]
- Martinez-Lozano Sinues, P.; Meier, L.; Berchtold, C.; Ivanov, M.; Sievi, N.; Camen, G.; Kohler, M.; Zenobi, R. Breath analysis in real time by mass spectrometry in chronic obstructive pulmonary disease. Respiration 2014, 87, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, D.; Keir, H.R.; Richardson, H.; Mayhew, D.; Boyer, J.; van der Schee, M.P.; Allsworth, M.D.; Miller, B.E.; Tal-Singer, R.; Chalmers, J.D. Exhaled volatile organic compounds and lung microbiome in copd: A pilot randomised controlled trial. ERJ Open Res. 2021, 7, 00253–2021. [Google Scholar] [CrossRef] [PubMed]
- Balfour-Lynn, I.M.; Laverty, A.; Dinwiddie, R. Reduced upper airway nitric oxide in cystic fibrosis. Arch. Dis. Child 1996, 75, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.R.; Kharitonov, S.A.; Scott, S.F.; Hodson, M.E.; Barnes, P.J. Nasal and exhaled nitric oxide is reduced in adult patients with cystic fibrosis and does not correlate with cystic fibrosis genotype. Chest 2000, 117, 1085–1089. [Google Scholar] [CrossRef] [Green Version]
- Kamboures, M.A.; Blake, D.R.; Cooper, D.M.; Newcomb, R.L.; Barker, M.; Larson, J.K.; Meinardi, S.; Nussbaum, E.; Rowland, F.S. Breath sulfides and pulmonary function in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2005, 102, 15762–15767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaisl, T.; Bregy, L.; Stebler, N.; Gaugg, M.T.; Bruderer, T.; Garcia-Gomez, D.; Moeller, A.; Singer, F.; Schwarz, E.I.; Benden, C.; et al. Real-time exhaled breath analysis in patients with cystic fibrosis and controls. J. Breath Res. 2018, 12, 036013. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Haas, N.; Baghdasaryan, A.; Bruderer, T.; Inci, D.; Micic, S.; Perkins, N.; Spinas, R.; Zenobi, R.; Moeller, A. Volatile organic compound breath signatures of children with cystic fibrosis by real-time sesi-hrms. ERJ Open Res. 2020, 6, 00171–2019. [Google Scholar] [CrossRef]
- Ibrahim, W.; Cordell, R.L.; Wilde, M.J.; Richardson, M.; Carr, L.; Sundari Devi Dasi, A.; Hargadon, B.; Free, R.C.; Monks, P.S.; Brightling, C.E.; et al. Diagnosis of COVID-19 by exhaled breath analysis using gas chromatography-mass spectrometry. ERJ Open Res. 2021, 7, 00139–2021. [Google Scholar] [CrossRef]
- Ruszkiewicz, D.M.; Sanders, D.; O’Brien, R.; Hempel, F.; Reed, M.J.; Riepe, A.C.; Bailie, K.; Brodrick, E.; Darnley, K.; Ellerkmann, R.; et al. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry—a feasibility study. EClinicalMedicine 2020, 29, 100609. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zieve, L.; Mahadevan, V. Mercaptans and dimethyl sulfide in the breath of patients with cirrhosis of the liver. Effect of feeding methionine. J. Lab. Clin. Med. 1970, 75, 628–635. [Google Scholar]
- Kaji, H.; Hisamura, M.; Saito, N.; Murao, M. Evaluation of volatile sulfur compounds in the expired alveolar gas in patients with liver cirrhosis. Clin. Chim. Acta 1978, 85, 279–284. [Google Scholar]
- Tangerman, A.; Meuwese-Arends, M.T.; Jansen, J.B. Cause and composition of foetor hepaticus. Lancet 1994, 343, 483. [Google Scholar] [CrossRef]
- Friedman, M.I.; Preti, G.; Deems, R.O.; Friedman, L.S.; Munoz, S.J.; Maddrey, W.C. Limonene in expired lung air of patients with liver disease. Dig. Dis. Sci. 1994, 39, 1672–1676. [Google Scholar] [CrossRef]
- Sehnert, S.S.; Jiang, L.; Burdick, J.F.; Risby, T.H. Breath biomarkers for detection of human liver diseases: Preliminary study. Biomarkers 2002, 7, 174–187. [Google Scholar] [CrossRef]
- Van den Velde, S.; Nevens, F.; Van Hee, P.; van Steenberghe, D.; Quirynen, M. Gc-ms analysis of breath odor compounds in liver patients. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 875, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Dadamio, J.; Van den Velde, S.; Laleman, W.; Van Hee, P.; Coucke, W.; Nevens, F.; Quirynen, M. Breath biomarkers of liver cirrhosis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 905, 17–22. [Google Scholar] [CrossRef]
- Pijls, K.E.; Smolinska, A.; Jonkers, D.M.; Dallinga, J.W.; Masclee, A.A.; Koek, G.H.; van Schooten, F.J. A profile of volatile organic compounds in exhaled air as a potential non-invasive biomarker for liver cirrhosis. Sci. Rep. 2016, 6, 19903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morisco, F.; Aprea, E.; Lembo, V.; Fogliano, V.; Vitaglione, P.; Mazzone, G.; Cappellin, L.; Gasperi, F.; Masone, S.; De Palma, G.D.; et al. Rapid “breath-print” of liver cirrhosis by proton transfer reaction time-of-flight mass spectrometry. A pilot study. PLoS ONE 2013, 8, e59658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez Del Rio, R.; O’Hara, M.E.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Volatile biomarkers in breath associated with liver cirrhosis—comparisons of pre- and post-liver transplant breath samples. EBioMedicine 2015, 2, 1243–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, R.; Lockman, K.A.; Homer, N.Z.M.; Bower, E.; Brinkman, P.; Knobel, H.H.; Fallowfield, J.A.; Jaap, A.J.; Hayes, P.C.; Plevris, J.N. Volatomic analysis identifies compounds that can stratify non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100137. [Google Scholar] [CrossRef]
- Ferrandino, G.; Orf, I.; Smith, R.; Calcagno, M.; Thind, A.K.; Debiram-Beecham, I.; Williams, M.; Gandelman, O.; de Saedeleer, A.; Kibble, G.; et al. Breath biopsy assessment of liver disease using an exogenous volatile organic compound-toward improved detection of liver impairment. Clin. Transl. Gastroenterol. 2020, 11, e00239. [Google Scholar] [CrossRef]
- Miyazawa, M.; Shindo, M.; Shimada, T. Metabolism of (+)- and (−)-limonenes to respective carveols and perillyl alcohols by cyp2c9 and cyp2c19 in human liver microsomes. Drug Metab. Dispos. 2002, 30, 602–607. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.A.; Hakim, I.A.; Chew, W.; Thompson, P.; Thomson, C.A.; Chow, H.H. Adipose tissue accumulation of d-limonene with the consumption of a lemonade preparation rich in d-limonene content. Nutr. Cancer 2010, 62, 783–788. [Google Scholar] [CrossRef] [PubMed]
- DiVincenzo, G.D.; Kaplan, C.J.; Dedinas, J. Characterization of the metabolites of methyl n-butyl ketone, methyl iso-butyl ketone, and methyl ethyl ketone in guinea pig serum and their clearance. Toxicol. Appl. Pharmacol. 1976, 36, 511–522. [Google Scholar] [CrossRef]
- Chiba, S.; Oshida, S. Metabolism and toxicity of n-pentane and isopentane. Nihon Hoigaku Zasshi 1991, 45, 128–137. [Google Scholar] [PubMed]
- Ahmed Laskar, A.; Younus, H. Aldehyde toxicity and metabolism: The role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab. Rev. 2019, 51, 42–64. [Google Scholar] [CrossRef]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry; W.H. Freeman: New York, NY, USA, 2005. [Google Scholar]
- Bingham, E.; Cohrssen, B.; Powell, C.H. Patty’s Toxicology, 5th ed.; Wiley: New York, NY, USA, 2001; pp. 1–9. [Google Scholar]
- Chan, L.W.; Anahtar, M.N.; Ong, T.H.; Hern, K.E.; Kunz, R.R.; Bhatia, S.N. Engineering synthetic breath biomarkers for respiratory disease. Nat. Nanotechnol. 2020, 15, 792–800. [Google Scholar] [CrossRef]
- Hardwick, R.N.; Fisher, C.D.; Street, S.M.; Canet, M.J.; Cherrington, N.J. Molecular mechanism of altered ezetimibe disposition in nonalcoholic steatohepatitis. Drug Metab. Dispos. 2012, 40, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Villeneuve, J.P.; Infante-Rivard, C.; Ampelas, M.; Pomier-Layrargues, G.; Huet, P.M.; Marleau, D. Prognostic value of the aminopyrine breath test in cirrhotic patients. Hepatology 1986, 6, 928–931. [Google Scholar] [CrossRef]
- Konstantinou, D.; Margariti, E.; Hadziyannis, E.; Pectasides, D.; Papatheodoridis, G.V. Significance of the (13)c-caffeine breath test for patients with cirrhosis. Ann. Gastroenterol. 2014, 27, 53–59. [Google Scholar] [PubMed]
- Braden, B.; Faust, D.; Sarrazin, U.; Zeuzem, S.; Dietrich, C.F.; Caspary, W.F.; Sarrazin, C. 13c-methacetin breath test as liver function test in patients with chronic hepatitis c virus infection. Aliment. Pharmacol. Ther. 2005, 21, 179–185. [Google Scholar] [CrossRef]
- Merkel, C.; Bolognesi, M.; Bellon, S.; Bianco, S.; Honisch, B.; Lampe, H.; Angeli, P.; Gatta, A. Aminopyrine breath test in the prognostic evaluation of patients with cirrhosis. Gut 1992, 33, 836–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, G.J.; Katelaris, P.H.; Jones, D.B.; Seow, F.; Le Couteur, D.G.; Ngu, M.C. Validity of the 13c-caffeine breath test as a noninvasive, quantitative test of liver function. Hepatology 2003, 38, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Schmilovitz-Weiss, H.; Niv, Y.; Pappo, O.; Halpern, M.; Sulkes, J.; Braun, M.; Barak, N.; Rotman, Y.; Cohen, M.; Waked, A.; et al. The 13c-caffeine breath test detects significant fibrosis in patients with nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2008, 42, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Park, G.J.; Wiseman, E.; George, J.; Katelaris, P.H.; Seow, F.; Fung, C.; Ngu, M.C. Non-invasive estimation of liver fibrosis in non-alcoholic fatty liver disease using the 13 c-caffeine breath test. J. Gastroenterol. Hepatol. 2011, 26, 1411–1416. [Google Scholar] [PubMed]
- Stockmann, M.; Lock, J.F.; Malinowski, M.; Niehues, S.M.; Seehofer, D.; Neuhaus, P. The limax test: A new liver function test for predicting postoperative outcome in liver surgery. HPB 2010, 12, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Bonfrate, L.; Grattagliano, I.; Palasciano, G.; Portincasa, P. Dynamic carbon 13 breath tests for the study of liver function and gastric emptying. Gastroenterol. Rep. 2015, 3, 12–21. [Google Scholar] [CrossRef]
- The-European-Commission. Commission implementing regulation (eu) no 872/2012 adopting the list of flavouring substances provided for by regulation (ec) no 2232/96 of the european parliament and of the council, introducing it in annex i to regulation (ec) no 1334/2008 of the european parliament and of the council and repealing commission regulation (ec) no 1565/2000 and commission decision 1999/217/ec. In Official Journal of the European Union; 2012; L 267/271. [Google Scholar]
- Hiki, N.; Kaminishi, M.; Hasunuma, T.; Nakamura, M.; Nomura, S.; Yahagi, N.; Tajiri, H.; Suzuki, H. A phase i study evaluating tolerability, pharmacokinetics, and preliminary efficacy of l-menthol in upper gastrointestinal endoscopy. Clin. Pharmacol. Ther. 2011, 90, 221–228. [Google Scholar] [CrossRef]
- Duisken, M.; Sandner, F.; Blomeke, B.; Hollender, J. Metabolism of 1,8-cineole by human cytochrome p450 enzymes: Identification of a new hydroxylated metabolite. Biochim. Biophys. Acta 2005, 1722, 304–311. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Poon, G.K.; Boddy, A.; English, J.; Halbert, G.W.; Pagonis, C.; Jarman, M.; Coombes, R.C. Phase i and pharmacokinetic study of d-limonene in patients with advanced cancer. Cancer research campaign phase i/ii clinical trials committee. Cancer Chemother. Pharmacol. 1998, 42, 111–117. [Google Scholar] [CrossRef]
- Wilkinson, M.; White, I.R.; Hamshere, K.; Holz, O.; Schuchardt, S.; Bellagambi, F.G.; Lomonaco, T.; Biagini, D.; Di Francesco, F.; Fowler, S.J. The peppermint breath test: A benchmarking protocol for breath sampling and analysis using gc-ms. J. Breath Res. 2020, 15, 026006. [Google Scholar]
- Henderson, B.; Ruszkiewicz, D.M.; Wilkinson, M.; Beauchamp, J.D.; Cristescu, S.M.; Fowler, S.J.; Salman, D.; Francesco, F.D.; Koppen, G.; Langejurgen, J.; et al. A benchmarking protocol for breath analysis: The peppermint experiment. J. Breath Res. 2020, 14, 046008. [Google Scholar] [CrossRef]
- Morey, T.E.; Booth, M.; Wasdo, S.; Wishin, J.; Quinn, B.; Gonzalez, D.; Derendorf, H.; McGorray, S.P.; Simoni, J.; Melker, R.J.; et al. Oral adherence monitoring using a breath test to supplement highly active antiretroviral therapy. AIDS Behav. 2013, 17, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Badrick, T.; Turner, P. Review and recommendations for the component tests in the liver function test profile. Indian J. Clin. Biochem. 2016, 31, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Zang, W.; Zhou, M.; Schafer, N.; Begley, L.A.; Huang, Y.J.; Fan, X. Real time breath analysis using portable gas chromatography for adult asthma phenotypes. Metabolites 2021, 11, 265. [Google Scholar] [CrossRef]
- Hoang, S.A.; Oseini, A.; Feaver, R.E.; Cole, B.K.; Asgharpour, A.; Vincent, R.; Siddiqui, M.; Lawson, M.J.; Day, N.C.; Taylor, J.M.; et al. Gene expression predicts histological severity and reveals distinct molecular profiles of nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 12541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amann, A.; Miekisch, W.; Schubert, J.; Buszewski, B.; Ligor, T.; Jezierski, T.; Pleil, J.; Risby, T. Analysis of exhaled breath for disease detection. Annu. Rev. Anal. Chem. 2014, 7, 455–482. [Google Scholar] [CrossRef] [PubMed]



| Author/Year | Study Design | Analytical Method | Discriminant VOCs | Calssification Performance |
|---|---|---|---|---|
| Friedman et al. (1994) [82] | 24 CLD 24 Controls | GC-MS | Hydrogen-sulphide ↑ Limonene ↑ | n/a |
| Sehnert et al. (2002) [83] | 86 CLD 109 Controls | GC | Carbonyl sulphide ↑ | n/a |
| Van den Velde et al. (2008) [84] | 52 CLD 50 Controls | GC-MS | Acetone ↑ Dimethyl-sulphide ↑ 2-butanone ↑ 2-pentanone ↑ Indole ↓ Dimethyl-selenide ↓ | 100% sensitivity 70% specificity |
| Dadamio et al. (2012) [85] | 35 CLD 49 Controls | GC-MS | Dimethyl-sulphide ↑ Acetone ↑ 2-butanone ↑ 2-pentanone ↑ Indole ↓ Phenol ↓ Dimethyl-selenide ↓ Isoprene ↑ Ethane ↑ Pentane ↑ | 83% sensitivity 100% specificity |
| Pijls et al. (2016) [86] | 34 cirrhotic 87 non-cirrhotic 31 controls | GC-MS | Dimethyl-sulphide ↑ Terpene (limonene) ↑ 2-methyl-butanal ↓ Propanoic acid ↑ Octane ↑ Terpenoid ↑ 3-carene ↑ 1-hexadecanol ↓ C16H34 ↓ | 83% sensitivity 87% specificity |
| Morisco et al. (2013) [87] | 12 CLD 14 Controls | PTR-MS |
Heptadienol ↑ Methanol ↑ 2-butanone ↑ 3-pentone ↑ 2-octanone ↑ 2-nonanone ↑ Monoterpene ↑ P-cymene ↑ | 83% sensitivity 86% specificity |
| Fernandez Del Rio et al. (2015) [88] | 31 CLD 30 Controls | PTR-MS | Methanol ↑ 2-butanone ↑ Carbon-sulphide ↑ 2-pentanone ↑ Limonene ↑ | 97% sensitivity 70% specificity |
| Sinha et al. (2020) [89] | 15 chirrosis NAFLD 14 non-cirrhosis NAFLD 14 Controls | GC-MS | Styrene Acetone Isoprene DMS D-limonene Acetophenone Terpinene | Cirrhotic vs. Control: AUCs = 0.98 Cirrhotic vs. Non-cirrhotic: AUC = 0.91 Non-Cirrhotic vs. Control: AUC = 0.84 |
| Ferrandino et al. [90] | 44 cirrhosis 42 controls | GC-MS | Limonene | AUC = 0.78 |
| Discriminant VOC | Dietary Sources | Metabolizing Pathway(s) | Main Bioproduct(s) |
|---|---|---|---|
| Limonene | Fruit, fruit juices, citrus products | CYP2C9/CYP2C19 [91] | perillyl alcohol [91] trans carveol |
| 2-butanone | Ripe fruit | Cytochrome P450 system [93] | 3-hydroxy-2-butanone and 2,3-butanediol [93] |
| 2-pentanone | Fruit, cheese, whiskey | Alcohol dehydrogenases (ADH) [94] ? | 2-pentanol [94] ? |
| 2-methyl-butanal | Crystal malts, baked potatoes, whole milk powder | Aldehyde dehydrogenases (ALDH) [95] | 2-methyl-butanol [95] |
| Propanoic acid | Butter, cheese | Propionyl-CoA carboxylase (PCC) [96] | D-methylmalonyl-CoA [96] |
| 1-hexadecanol | palm or coconut oil | Cytochrome P450 system [97] | Palmitic acid [97] |
| Acetophenone | Fruit | Glucuronosyltransferase (UGT) hippuric acid metabolism [97] | 1-phenylethanol-glucoronide hippuric acid [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgia, A.; Ahmed, Y.; Sweeney, K.; Nicholson-Scott, L.; Arthur, K.; Allsworth, M.; Boyle, B.; Gandelman, O.; Smolinska, A.; Ferrandino, G. Breath-Taking Perspectives and Preliminary Data toward Early Detection of Chronic Liver Diseases. Biomedicines 2021, 9, 1563. https://doi.org/10.3390/biomedicines9111563
Murgia A, Ahmed Y, Sweeney K, Nicholson-Scott L, Arthur K, Allsworth M, Boyle B, Gandelman O, Smolinska A, Ferrandino G. Breath-Taking Perspectives and Preliminary Data toward Early Detection of Chronic Liver Diseases. Biomedicines. 2021; 9(11):1563. https://doi.org/10.3390/biomedicines9111563
Chicago/Turabian StyleMurgia, Antonio, Yusuf Ahmed, Kelly Sweeney, Louise Nicholson-Scott, Kayleigh Arthur, Max Allsworth, Billy Boyle, Olga Gandelman, Agnieszka Smolinska, and Giuseppe Ferrandino. 2021. "Breath-Taking Perspectives and Preliminary Data toward Early Detection of Chronic Liver Diseases" Biomedicines 9, no. 11: 1563. https://doi.org/10.3390/biomedicines9111563
APA StyleMurgia, A., Ahmed, Y., Sweeney, K., Nicholson-Scott, L., Arthur, K., Allsworth, M., Boyle, B., Gandelman, O., Smolinska, A., & Ferrandino, G. (2021). Breath-Taking Perspectives and Preliminary Data toward Early Detection of Chronic Liver Diseases. Biomedicines, 9(11), 1563. https://doi.org/10.3390/biomedicines9111563