A Novel Cellular Therapy to Treat Pancreatic Pain in Experimental Chronic Pancreatitis Using Human Alpha-1 Antitrypsin Overexpressing Mesenchymal Stromal Cells
Abstract
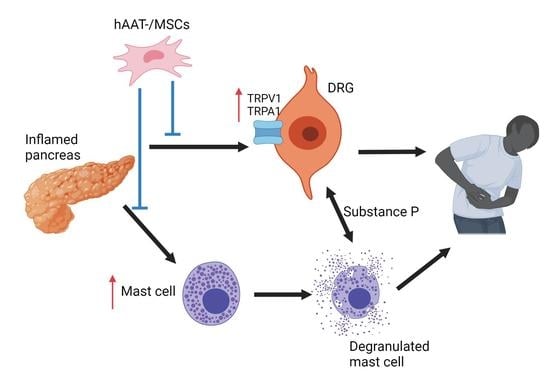
:1. Introduction
2. Materials and Methods
2.1. Animal
2.2. hAAT-MSC and MSC Preparation and Infusion
2.3. Histological Scoring of the Pancreas
- (1)
- total area preserved (0%—the whole pancreas is damaged to 100%—no damage in the pancreas)
- (2)
- inflammation (0—no inflammation; 1—less than 25% inflammation; 2—25–50% inflammation; 3—50–75% inflammation and 4–100% inflammation)
- (3)
- necrosis (0—no necrosis; 1—minimal necrosis; 2—mild necrosis; 3—moderate necrosis; 4—severe necrosis)
- (4)
- fibrosis (0—no fibrosis; 1—minimal fibrosis; 2—mild fibrosis; 3—moderate fibrosis; 4—severe fibrosis)
- (5)
- vacuole formation in acinar cells (0—no vacuolization; 1—less than 25% vacuolization; 2—25–50% vacuolization; 3—50–75% vacuolization and 4—100% vacuolization)
- (6)
- interlobular edema (0—no interlobular edema; 1—minimal interlobular edema; 2—mild interlobular edema; 3—moderate interlobular edema; 4—severe interlobular edema)
2.4. May-Grünwald-Giemsa Staining for Detection of Mast Cells
2.5. Immunohistochemistry
2.6. Behavioral Assessment of Pain
2.7. Statistical Analyses
3. Results
3.1. Mice Treated with MSCs or hAAT-MSCs Show Less Pancreatic Injury after TNBS Infusion
3.2. MSC Infusion Preserves Pancreatic Histology in CP Mice
3.3. MSC Infusion Ameliorates CP Pain
3.4. MSC Pain Reduction Is Associated with Downregulation of TRPV1 Expression
3.5. MSC Infusions Inhibit Mast Cell Infiltration into the Pancreas of CP Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Ouyang, G.; Pan, G.; Liu, Q.; Wu, Y.; Liu, Z.; Lu, W.; Li, S.; Zhou, Z.; Wen, Y. The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. BMC Med. 2020, 18, 388. [Google Scholar] [CrossRef]
- Lew, D.; Afghani, E.; Pandol, S. Chronic Pancreatitis: Current Status and Challenges for Prevention and Treatment. Dig. Dis. Sci. 2017, 62, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Goulden, M.R. The pain of chronic pancreatitis: A persistent clinical challenge. Br. J. Pain 2013, 7, 8–22. [Google Scholar] [CrossRef] [Green Version]
- Bouwense, S.A.W.; Kempeneers, M.A.; van Santvoort, H.C.; Boermeester, M.A.; van Goor, H.; Besselink, M.G. Surgery in Chronic Pancreatitis: Indication, Timing and Procedures. Visc. Med. 2019, 35, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Tieftrunk, E.; Maak, M.; Friess, H.; Ceyhan, G.O. Pain mechanisms in chronic pancreatitis: Of a master and his fire. Langenbecks Arch. Surg. 2011, 396, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Tieftrunk, E.; Demir, I.E.; Simon, P.; Friess, H.; Ceyhan, G.O. Evidence of pancreatic neuropathy and neuropathic pain in hereditary chronic pancreatitis. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2013, 13, 629–630. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, J.G.; Hartel, M.; Demir, I.E.; Hinz, U.; Bergmann, F.; Buchler, M.W.; Friess, H.; Ceyhan, G.O. Pain sensation in pancreatic diseases is not uniform: The different facets of pancreatic pain. World J. Gastroenterol. 2014, 20, 9154–9161. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, J.L.; Olesen, S.S.; Malver, L.P.; Frøkjær, J.B.; Drewes, A.M. Pain and chronic pancreatitis: A complex interplay of multiple mechanisms. World J. Gastroenterol. 2013, 19, 7282–7291. [Google Scholar] [CrossRef]
- Phinney, D.G.; Isakova, I. Plasticity and Therapeutic Potential of Mesenchymal Stem Cells in the Nervous System. Curr. Pharm. Des. 2005, 11, 1255–1265. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentis, J.; Sanchez, A.; Garcia-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Bin Abdullah, B.J.; Dhillon, S.S.; Vijanari, A.; Anoop, C.H.; Gupta, P.K. Intra-arterial allogeneic mesenchymal stem cells for critical limb ischemia are safe and efficacious: Report of a phase I study. World J. Surg. 2013, 37, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Siniscalco, D.; Giordano, C.; Galderisi, U.; Luongo, L.; de Novellis, V.; Rossi, F.; Maione, S. Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Front. Integr. Neurosci. 2011, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- Naruse, K.; Sato, J.; Funakubo, M.; Hata, M.; Nakamura, N.; Kobayashi, Y.; Kamiya, H.; Shibata, T.; Kondo, M.; Himeno, T.; et al. Transplantation of bone marrow-derived mononuclear cells improves mechanical hyperalgesia, cold allodynia and nerve function in diabetic neuropathy. PLoS ONE 2011, 6, e27458. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Wang, H.; Zou, S.; Gu, M.; Watanabe, M.; Wei, F.; Dubner, R.; Huang, G.T.; Ren, K. Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells 2011, 29, 1294–1303. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.H.; Song, S.U.; Yi, T.; Jeon, M.S.; Hong, S.W.; Zheng, H.M.; Lee, H.S.; Choi, M.J.; Lee, D.H.; Hong, S.S. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology 2011, 140, 998–1008. [Google Scholar] [CrossRef]
- Kawakubo, K.; Ohnishi, S.; Fujita, H.; Kuwatani, M.; Onishi, R.; Masamune, A.; Takeda, H.; Sakamoto, N. Effect of Fetal Membrane-Derived Mesenchymal Stem Cell Transplantation in Rats with Acute and Chronic Pancreatitis. Pancreas 2016, 45, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Nakhleh, R.E.; Snover, D.C. Use of alpha-1-antitrypsin staining in the diagnosis of nodular regenerative hyperplasia of the liver. Hum. Pathol. 1988, 19, 1048–1052. [Google Scholar] [CrossRef]
- Pott, G.B.; Chan, E.D.; Dinarello, C.A.; Shapiro, L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J. Leukoc. Biol. 2009, 85, 886–895. [Google Scholar] [CrossRef] [Green Version]
- Bucurenci, N.; Blake, D.R.; Chidwick, K.; Winyard, P.G. Inhibition of neutrophil superoxide production by human plasma alpha 1-antitrypsin. FEBS Lett. 1992, 300, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Akaike, T.; Alam, M.S.; Inoue, K.; Hamamoto, T.; Ikebe, N.; Yoshitake, J.; Okamoto, T.; Maeda, H. Novel functions of human alpha(1)-protease inhibitor after S-nitrosylation: Inhibition of cysteine protease and antibacterial activity. Biochem. Biophys. Res. Commun. 2000, 267, 918–923. [Google Scholar] [CrossRef]
- Koulmanda, M.; Bhasin, M.; Hoffman, L.; Fan, Z.; Qipo, A.; Shi, H.; Bonner-Weir, S.; Putheti, P.; Degauque, N.; Libermann, T.A.; et al. Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc. Natl. Acad. Sci. USA 2008, 105, 16242–16247. [Google Scholar] [CrossRef] [Green Version]
- Aldonyte, R.; Hutchinson, T.E.; Jin, B.; Brantly, M.; Block, E.; Patel, J.; Zhang, J. Endothelial alpha-1-antitrypsin attenuates cigarette smoke induced apoptosis in vitro. COPD 2008, 5, 153–162. [Google Scholar] [CrossRef]
- Dabbagh, K.; Laurent, G.J.; Shock, A.; Leoni, P.; Papakrivopoulou, J.; Chambers, R.C. Alpha-1-antitrypsin stimulates fibroblast proliferation and procollagen production and activates classical MAP kinase signalling pathways. J. Cell Physiol. 2001, 186, 73–81. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Oton-Gonzalez, L.; Selvatici, R.; Rizzo, P.; Pavasini, R.; Campo, G.C.; Lanzillotti, C.; Mazziotta, C.; De Mattei, M.; Tognon, M.; et al. SERPINA1 Gene Promoter Is Differentially Methylated in Peripheral Blood Mononuclear Cells of Pregnant Women. Front. Cell Dev. Biol. 2020, 8, 550543. [Google Scholar] [CrossRef]
- Cao, J.J.; Gregoire, B.R.; Sun, L.; Song, S. Alpha-1 antitrypsin reduces ovariectomy-induced bone loss in mice. Ann. N. Y. Acad. Sci. 2011, 1240, E31–E35. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Gou, W.; Wang, J.; Wei, H.; Lee, J.; Strange, C.; Wang, H. Overexpression of alpha-1 antitrypsin in mesenchymal stromal cells improves their intrinsic biological properties and therapeutic effects in nonobese diabetic mice. Stem Cells Transl. Med. 2021, 10, 320–331. [Google Scholar] [CrossRef]
- Gou, W.; Swaby, L.; Wolfe, A.M.; Lancaster, W.P.; Morgan, K.A.; Wang, H. A Mouse Model for Chronic Pancreatitis via Bile Duct TNBS Infusion. J. Vis. Exp. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cattaruzza, F.; Johnson, C.; Leggit, A.; Grady, E.; Schenk, A.K.; Cevikbas, F.; Cedron, W.; Bondada, S.; Kirkwood, R.; Malone, B.; et al. Transient receptor potential ankyrin 1 mediates chronic pancreatitis pain in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G1002–G1012. [Google Scholar] [CrossRef] [Green Version]
- Puig-Diví, V.; Molero, X.; Salas, A.; Guarner, F.; Guarner, L.; Malagelada, J.R. Induction of chronic pancreatic disease by trinitrobenzene sulfonic acid infusion into rat pancreatic ducts. Pancreas 1996, 13, 417–424. [Google Scholar] [CrossRef]
- Winston, J.H.; He, Z.J.; Shenoy, M.; Xiao, S.Y.; Pasricha, P.J. Molecular and behavioral changes in nociception in a novel rat model of chronic pancreatitis for the study of pain. Pain 2005, 117, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Adrian, T. Chronic pancreatitis. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–6. [Google Scholar]
- Aghdassi, A.A.; Mayerle, J.; Christochowitz, S.; Weiss, F.U.; Sendler, M.; Lerch, M.M. Animal models for investigating chronic pancreatitis. Fibrogenes. Tissue Repair. 2011, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerch, M.M.; Gorelick, F.S. Models of acute and chronic pancreatitis. Gastroenterology 2013, 144, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.A.; Kwok, L.W.; Hovav, A.H.; Ohle, S.J.; Little, F.F.; Fine, A.; Kotton, D.N. Sustained expression of alpha1-antitrypsin after transplantation of manipulated hematopoietic stem cells. Am. J. Respir. Cell Mol. Biol. 2008, 39, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Fukumura, Y. Histological Characteristics of Chronic Pancreatitis, Based upon Etiology. Pancreas Pathol. Pract. Res. 2007, 146–153. [Google Scholar] [CrossRef]
- Mutsaddi, S.; Kotrashetti, V.S.; Nayak, R.S.; Pattanshetty, S.M. Comparison of histochemical staining techniques for detecting mast cells in oral lesions. Biotech. Histochem. 2019, 94, 459–468. [Google Scholar] [CrossRef]
- Beaudry, K.L.; Parsons, C.L.M.; Ellis, S.E.; Akers, R.M. Localization and quantitation of macrophages, mast cells, and eosinophils in the developing bovine mammary gland1. J. Dairy Sci. 2016, 99, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Sleigh, J.N.; Weir, G.A.; Schiavo, G. A simple, step-by-step dissection protocol for the rapid isolation of mouse dorsal root ganglia. BMC Res. Notes 2016, 9, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMackin, M.Z.; Lewin, M.R.; Tabuena, D.R.; Arreola, F.E.; Moffatt, C.; Fuse, M. Use of von Frey filaments to assess nociceptive sensitization in the hornworm, Manduca sexta. J. Neurosci. Methods 2016, 257, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Vera-Portocarrero, L.P.; Lu, Y.; Westlund, K.N. Nociception in persistent pancreatitis in rats: Effects of morphine and neuropeptide alterations. Anesthesiology 2003, 98, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Dodda, D.; Chhajed, R.; Mishra, J.; Padhy, M. Targeting oxidative stress attenuates trinitrobenzene sulphonic acid induced inflammatory bowel disease like symptoms in rats: Role of quercetin. Indian J. Pharmacol. 2014, 46, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badiavas, E.V.; Abedi, M.; Butmarc, J.; Falanga, V.; Quesenberry, P. Participation of bone marrow derived cells in cutaneous wound healing. J. Cell Physiol. 2003, 196, 245–250. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Gambelli, F.; McBride, C.; Gaupp, D.; Baddoo, M.; Kaminski, N.; Phinney, D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl. Acad. Sci. USA 2003, 100, 8407–8411. [Google Scholar] [CrossRef] [Green Version]
- Minguell, J.J.; Erices, A. Mesenchymal stem cells and the treatment of cardiac disease. Exp. Biol. Med. 2006, 231, 39–49. [Google Scholar] [CrossRef]
- Xu, X.; Yu, H.; Sun, L.; Zheng, C.; Shan, Y.; Zhou, Z.; Wang, C.; Chen, B. Adipose-derived mesenchymal stem cells ameliorate dibutyltin dichloride-induced chronic pancreatitis by inhibiting the PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 2020, 21, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Gou, W.; Kim, D.S.; Dong, X.; Strange, C.; Tan, Y.; Adams, D.B.; Wang, H. Adipose Stem Cell Therapy Mitigates Chronic Pancreatitis via Differentiation into Acinar-like Cells in Mice. Mol. Ther. 2017, 25, 2490–2501. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Liu, C.; Zhang, H.; Pan, Y.; Tang, Q.; Liu, J.; Wang, Y.; Hu, M.; Xue, F. Effect of the IκBα mutant gene delivery to mesenchymal stem cells on rat chronic pancreatitis. Genet. Mol. Res. 2014, 13, 371–385. [Google Scholar] [CrossRef]
- Steinkohl, E.; Olesen, S.S.; Drewes, A.M.; Frøkjaer, J.B. Progression of pancreatic morphology in chronic pancreatitis is not associated with changes in quality of life and pain. Scand. J. Gastroenterol. 2020, 55, 1099–1107. [Google Scholar] [CrossRef]
- Zhou, C.H.; Li, M.L.; Qin, A.L.; Lv, S.X.; Wen, T.; Zhu, X.Y.; Li, L.Y.; Dong, Y.; Hu, C.Y.; Hu, D.M.; et al. Reduction of fibrosis in dibutyltin dichloride-induced chronic pancreatitis using rat umbilical mesenchymal stem cells from Wharton’s jelly. Pancreas 2013, 42, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.S.; La, J.H.; Scheff, N.N.; Davis, B.M.; Albers, K.M.; Gebhart, G.F. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J. Neurosci. 2013, 33, 5603–5611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liddle, R.A. The role of Transient Receptor Potential Vanilloid 1 (TRPV1) channels in pancreatitis. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 869–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, J.D.; Patel, A.A.; McVey, D.C.; Thomas, J.E.; Prpic, V.; Vigna, S.R.; Liddle, R.A. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1322–G1328. [Google Scholar] [CrossRef]
- Schwartz, E.S.; Christianson, J.A.; Chen, X.; La, J.H.; Davis, B.M.; Albers, K.M.; Gebhart, G.F. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology 2011, 140, 1283–1291.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, S.S.; Krauss, T.; Demir, I.E.; Wilder-Smith, O.H.; Ceyhan, G.O.; Pasricha, P.J.; Drewes, A.M. Towards a neurobiological understanding of pain in chronic pancreatitis: Mechanisms and implications for treatment. Pain Rep. 2017, 2, e625. [Google Scholar] [CrossRef]
- Brini, A.T.; Amodeo, G.; Ferreira, L.M.; Milani, A.; Niada, S.; Moschetti, G.; Franchi, S.; Borsani, E.; Rodella, L.F.; Panerai, A.E.; et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci. Rep. 2017, 7, 9904. [Google Scholar] [CrossRef] [Green Version]
- Mukhamedshina, Y.O.; Gracheva, O.A.; Mukhutdinova, D.M.; Chelyshev, Y.A.; Rizvanov, A.A. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen. Res. 2019, 14, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhu, J.; Zhu, M.; Wei, K.; Chen, Q.; Wu, X.; Miao, X.; Lu, Z. Inhibition of Mast Cell Degranulation Relieves Visceral Hypersensitivity Induced by Pancreatic Carcinoma in Mice. J. Mol. Neurosci. 2019, 69, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.J.; Zhang, Y.L.; Liu, L.; Pan, L.; Yao, S.K. Increased expression of nerve growth factor correlates with visceral hypersensitivity and impaired gut barrier function in diarrhoea-predominant irritable bowel syndrome: A preliminary explorative study. Aliment. Pharmacol. Ther. 2017, 45, 100–114. [Google Scholar] [CrossRef]
- Demir, I.E.; Schorn, S.; Schremmer-Danninger, E.; Wang, K.; Kehl, T.; Giese, N.A.; Algül, H.; Friess, H.; Ceyhan, G.O. Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS ONE 2013, 8, e60529. [Google Scholar] [CrossRef]
- Bicer, F.; Altuntas, C.Z.; Izgi, K.; Ozer, A.; Kavran, M.; Tuohy, V.K.; Daneshgari, F. Chronic pelvic allodynia is mediated by CCL2 through mast cells in an experimental autoimmune cystitis model. Am. J. Physiol. Ren. Physiol. 2014, 308, F103–F113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, D. What activates visceral afferents? Gut 2004, 53 (Suppl. S2), ii5–ii8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.M.; Nemeth, K.; Kushnir-Sukhov, N.M.; Metcalfe, D.D.; Mezey, E. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin. Exp. Allergy 2011, 41, 526–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazari, M.; Ni, N.C.; Lüdke, A.; Li, S.-H.; Guo, J.; Weisel, R.D.; Li, R.-K. Mast cells promote proliferation and migration and inhibit differentiation of mesenchymal stem cells through PDGF. J. Mol. Cell. Cardiol. 2016, 94, 32–42. [Google Scholar] [CrossRef]
- Wang, L.T.; Ting, C.H.; Yen, M.L.; Liu, K.J.; Sytwu, H.K.; Wu, K.K.; Yen, B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. J. Biomed. Sci. 2016, 23, 76. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.F.; O’Hara, M.D.; Laptev, A.V.; Halford, K.W.; Pollard, M.D.; Class, R.; Simon, D.; Livezey, K.; Prockop, D.J. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA 1998, 95, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Fischbein, M.P.; Robbins, R.C.; Pelletier, M.P. Stem cell transplantation: The lung barrier. Transpl. Proc. 2007, 39, 573–576. [Google Scholar] [CrossRef]
- Erkers, T.; Kaipe, H.; Nava, S.; Molldén, P.; Gustafsson, B.; Axelsson, R.; Ringdén, O. Treatment of severe chronic graft-versus-host disease with decidual stromal cells and tracing with (111)indium radiolabeling. Stem Cells Dev. 2015, 24, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholamrezanezhad, A.; Mirpour, S.; Bagheri, M.; Mohamadnejad, M.; Alimoghaddam, K.; Abdolahzadeh, L.; Saghari, M.; Malekzadeh, R. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl. Med. Biol. 2011, 38, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Olmo, D.; Schwartz, D.A. Cumulative Evidence That Mesenchymal Stem Cells Promote Healing of Perianal Fistulas of Patients with Crohn’s Disease-Going from Bench to Bedside. Gastroenterology 2015, 149, 853–857. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, R.P.; Nguyen, K.; Gou, W.; Green, E.; Morgan, K.; Lancaster, W.; Helke, K.; Strange, C.; Wang, H. A Novel Cellular Therapy to Treat Pancreatic Pain in Experimental Chronic Pancreatitis Using Human Alpha-1 Antitrypsin Overexpressing Mesenchymal Stromal Cells. Biomedicines 2021, 9, 1695. https://doi.org/10.3390/biomedicines9111695
Chow RP, Nguyen K, Gou W, Green E, Morgan K, Lancaster W, Helke K, Strange C, Wang H. A Novel Cellular Therapy to Treat Pancreatic Pain in Experimental Chronic Pancreatitis Using Human Alpha-1 Antitrypsin Overexpressing Mesenchymal Stromal Cells. Biomedicines. 2021; 9(11):1695. https://doi.org/10.3390/biomedicines9111695
Chicago/Turabian StyleChow, Rebecca P., Kevin Nguyen, Wenyu Gou, Erica Green, Katherine Morgan, William Lancaster, Kristi Helke, Charlie Strange, and Hongjun Wang. 2021. "A Novel Cellular Therapy to Treat Pancreatic Pain in Experimental Chronic Pancreatitis Using Human Alpha-1 Antitrypsin Overexpressing Mesenchymal Stromal Cells" Biomedicines 9, no. 11: 1695. https://doi.org/10.3390/biomedicines9111695
APA StyleChow, R. P., Nguyen, K., Gou, W., Green, E., Morgan, K., Lancaster, W., Helke, K., Strange, C., & Wang, H. (2021). A Novel Cellular Therapy to Treat Pancreatic Pain in Experimental Chronic Pancreatitis Using Human Alpha-1 Antitrypsin Overexpressing Mesenchymal Stromal Cells. Biomedicines, 9(11), 1695. https://doi.org/10.3390/biomedicines9111695