Relationship between Vitamin D Status and Antibody Response to COVID-19 mRNA Vaccination in Healthy Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Cohort
2.2. SARS-CoV-2 IgG Measurement
2.3. Diagnostics of Neutralising Antibodies
2.4. 25-Hydroxyvitamin D Measurement
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. SARS-CoV-2 IgG Response and Neutralisation Potency following Vaccination
3.3. IgG Titres and Respective Neutralisation Potency in Relation to Age
3.4. Vitamin D Status over the Course of the Study
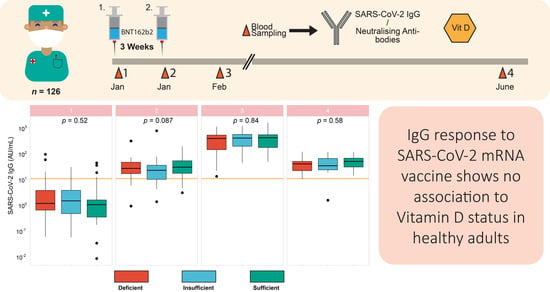
3.5. Vitamin D Status in Relation to Vaccination Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- da Silva Toscano, G.A.; de Araújo, I.I.; de Souza, T.A.; Barbosa Mirabal, I.R.; de Vasconcelos Torres, G. Vitamin C and D supplementation and the severity of COVID-19: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021, 100, e26427. [Google Scholar] [CrossRef]
- Lordan, R.; Rando, H.M.; Consortium, C.-R.; Greene, C.S. Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. mSystems 2021, 6, e00122-21. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19-A Preventable Trigger for Autoimmune Disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef] [PubMed]
- Prothero, L. Ambulance staff awareness of vitamin D and risk of deficiency in a UK ambulance service: A survey-based evaluation. Br. Paramed. J. 2021, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H. Vitamin D Supplementation to Prevent COVID-19 Infections and Deaths-Accumulating Evidence from Epidemiological and Intervention Studies Calls for Immediate Action. Nutrients 2021, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubee, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Verdoia, M.; De Luca, G. Potential role of hypovitaminosis D and vitamin D supplementation during COVID-19 pandemic. QJM 2021, 114, 3–10. [Google Scholar] [CrossRef]
- Chiu, S.K.; Tsai, K.W.; Wu, C.C.; Zheng, C.M.; Yang, C.H.; Hu, W.C.; Hou, Y.C.; Lu, K.C.; Chao, Y.C. Putative Role of Vitamin D for COVID-19 Vaccination. Int. J. Mol. Sci. 2021, 22, 8988. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Luciani, A.; Perego, G.; Dognini, G.; Colombelli, P.L.; Ghidini, A. Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies. J. Steroid Biochem. Mol. Biol. 2021, 211, 105883. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D and Its Potential Benefit for the COVID-19 Pandemic. Endocr. Pract. 2021, 27, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Bogliolo, L.; de Stefano, L.; Caccialanza, R. A brief discussion of the benefit and mechanism of vitamin D supplementation on coronavirus disease 2019. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 102–107. [Google Scholar] [CrossRef]
- Schoenmakers, I.; Fraser, W.D.; Forbes, A. Vitamin D and acute and severe illness—a mechanistic and pharmacokinetic perspective. Nutr. Res. Rev. 2021, 1–16. [Google Scholar] [CrossRef]
- Jude, E.B.; Ling, S.F.; Allcock, R.; Yeap, B.X.Y.; Pappachan, J.M. Vitamin D deficiency is associated with higher hospitalisation risk from COVID-19: A retrospective case-control study. J. Clin. Endocrinol. Metab. 2021, 106, e4708–e4715. [Google Scholar] [CrossRef]
- Liu, N.; Sun, J.; Wang, X.; Zhang, T.; Zhao, M.; Li, H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: A systematic review and meta-analysis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 104, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Sooriyaarachchi, P.; Jeyakumar, D.T.; King, N.; Jayawardena, R. Impact of vitamin D deficiency on COVID-19. Clin. Nutr. ESPEN 2021, 44, 372–378. [Google Scholar] [CrossRef]
- Drame, M.; Cofais, C.; Hentzien, M.; Proye, E.; Coulibaly, P.S.; Demoustier-Tampere, D.; Destailleur, M.H.; Lotin, M.; Cantagrit, E.; Cebille, A.; et al. Relation between Vitamin D and COVID-19 in Aged People: A Systematic Review. Nutrients 2021, 13, 1339. [Google Scholar] [CrossRef]
- Cozier, Y.C.; Castro-Webb, N.; Hochberg, N.S.; Rosenberg, L.; Albert, M.A.; Palmer, J.R. Lower serum 25(OH)D levels associated with higher risk of COVID-19 infection in U.S. Black women. PLoS ONE 2021, 16, e0255132. [Google Scholar] [CrossRef]
- Sinaci, S.; Ocal, D.F.; Yucel Yetiskin, D.F.; Uyan Hendem, D.; Buyuk, G.N.; Goncu Ayhan, S.; Tanacan, A.; Ozgu-Erdinc, A.S.; Moraloglu Tekin, O.; Sahin, D. Impact of vitamin D on the course of COVID-19 during pregnancy: A case control study. J. Steroid Biochem. Mol. Biol. 2021, 213, 105964. [Google Scholar] [CrossRef] [PubMed]
- Oristrell, J.; Oliva, J.C.; Subirana, I.; Casado, E.; Dominguez, D.; Toloba, A.; Aguilera, P.; Esplugues, J.; Fafian, P.; Grau, M. Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study. Biomedicines 2021, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Chetty, V.V.; Chetty, M. Potential benefit of vitamin d supplementation in people with respiratory illnesses, during the Covid-19 pandemic. Clin. Transl. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eltriki, M.; Hopefl, R.; Wright, J.M.; Deb, S. Association between Vitamin D Status and Risk of Developing Severe COVID-19 Infection: A Meta-Analysis of Observational Studies. J. Am. Coll. Nutr. 2021, 1–11. [Google Scholar] [CrossRef]
- Ghasemian, R.; Shamshirian, A.; Heydari, K.; Malekan, M.; Alizadeh-Navaei, R.; Ebrahimzadeh, M.A.; Ebrahimi Warkiani, M.; Jafarpour, H.; Razavi Bazaz, S.; Rezaei Shahmirzadi, A.; et al. The role of vitamin D in the age of COVID-19: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14675. [Google Scholar] [CrossRef] [PubMed]
- Aloia, J.F.; Li-Ng, M. Re: Epidemic influenza and vitamin D. Epidemiol. Infect. 2007, 135, 1095–1096, author reply 1097–1098. [Google Scholar] [CrossRef] [Green Version]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, D.A.; Camargo, C.A., Jr.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Dominguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J. Endocrinol. Investig. 2021, 1–13. [Google Scholar] [CrossRef]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170. [Google Scholar] [CrossRef]
- Schuetz, P.; Gregoriano, C.; Keller, U. Supplementation of the population during the COVID-19 pandemic with vitamins and micronutrients—how much evidence is needed? Swiss Med. Wkly. 2021, 151, w20522. [Google Scholar] [CrossRef]
- Annweiler, C.; Beaudenon, M.; Simon, R.; Guenet, M.; Otekpo, M.; Celarier, T.; Gautier, J.; GERIA-COVID study group. Vitamin D supplementation prior to or during COVID-19 associated with better 3-month survival in geriatric patients: Extension phase of the GERIA-COVID study. J. Steroid Biochem. Mol. Biol. 2021, 213, 105958. [Google Scholar] [CrossRef]
- Nabi-Afjadi, M.; Karami, H.; Goudarzi, K.; Alipourfard, I.; Bahreini, E. The effect of vitamin D, magnesium and zinc supplements on interferon signaling pathways and their relationship to control SARS-CoV-2 infection. Clin. Mol. Allergy 2021, 19, 21. [Google Scholar] [CrossRef]
- Abobaker, A.; Alzwi, A.; Alraied, A.H.A. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol. Rep. 2020, 72, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Ghelani, D.; Alesi, S.; Mousa, A. Vitamin D and COVID-19: An Overview of Recent Evidence. Int. J. Mol. Sci. 2021, 22, 10559. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Vitamin D in the Healthy European Paediatric Population. J. Pediatric Gastroenterol. Nutr. 2013, 56, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H.; Henry, L. Tidyr: Easily Tidy Data with ‘Spread ()’ and ‘Gather ()’ Functions; R Package Version 0.8; 2018; Volume 2. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 14 November 2021).
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation; R Package Version 0.4; 2015; Volume 3, p. 156. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 14 November 2021).
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Jalkanen, P.; Kolehmainen, P.; Hakkinen, H.K.; Huttunen, M.; Tahtinen, P.A.; Lundberg, R.; Maljanen, S.; Reinholm, A.; Tauriainen, S.; Pakkanen, S.H.; et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021, 12, 3991. [Google Scholar] [CrossRef]
- Pegu, A.; O’Connell, S.E.; Schmidt, S.D.; O’Dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the elderly: The challenge of immune changes with aging. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef]
- Marot, S.; Malet, I.; Leducq, V.; Zafilaza, K.; Sterlin, D.; Planas, D.; Gothland, A.; Jary, A.; Dorgham, K.; Bruel, T.; et al. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat. Commun. 2021, 12, 2824. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Medical Advisory, S. Clinical utility of vitamin d testing: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2010, 10, 1–93. [Google Scholar]
- Aoun, A.; Maalouf, J.; Fahed, M.; El Jabbour, F. When and How to Diagnose and Treat Vitamin D Deficiency in Adults: A Practical and Clinical Update. J. Diet. Suppl. 2020, 17, 336–354. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Lambrinou, C.P.; Mavrogianni, C.; Tsirigoti, L.; Hoeller, U.; Roos, F.F.; Bendik, I.; Eggersdorfer, M.; Celis-Morales, C.; et al. Associations of vitamin D status with dietary intakes and physical activity levels among adults from seven European countries: The Food4Me study. Eur. J. Nutr. 2018, 57, 1357–1368. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, U.; Riedel, A.; Hirche, F.; Brandsch, C.; Girndt, M.; Ulrich, C.; Seibert, E.; Henning, C.; Glomb, M.A.; Dierkes, J.; et al. Vitamin D3 supplementation: Response and predictors of vitamin D3 metabolites—A randomized controlled trial. Clin. Nutr. 2016, 35, 351–358. [Google Scholar] [CrossRef]
- Slawin, A.; Brydak, L.B.; Doniec, Z.; Bujnowska-Fedak, M.; Mastalerz-Migas, A. Serum Vitamin D and Immunogenicity of Influenza Vaccination in the Elderly. Adv. Exp. Med. Biol. 2021, 1324, 21–28. [Google Scholar] [CrossRef]
- Lee, R.U.; Won, S.H.; Hansen, C.; Crum-Cianflone, N.F. 25-hydroxyvitamin D, influenza vaccine response and healthcare encounters among a young adult population. PLoS ONE 2018, 13, e0192479. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, M.E.; Talbot, H.K.; Zhu, Y.; Griffin, M.R.; Spencer, S.; Shay, D.K.; Coleman, L.A. Vitamin D is not associated with serologic response to influenza vaccine in adults over 50 years old. Vaccine 2013, 31, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-Mendes, N.; Talvas, J.; Dualé, C.; Guttmann, A.; Corbin, V.; Marceau, G.; Sapin, V.; Brachet, P.; Evrard, B.; Laurichesse, H.; et al. Impact of Vitamin D Supplementation on Influenza Vaccine Response and Immune Functions in Deficient Elderly Persons: A Randomized Placebo-Controlled Trial. Front. Immunol. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriesel, J.D.; Spruance, J. Calcitriol (1,25-dihydroxy-vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine 1999, 17, 1883–1888. [Google Scholar] [CrossRef]
- Peelen, E.; Rijkers, G.; Meerveld-Eggink, A.; Meijvis, S.; Vogt, M.; Cohen Tervaert, J.W.; Hupperts, R.; Damoiseaux, J. Relatively high serum vitamin D levels do not impair the antibody response to encapsulated bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 61–69. [Google Scholar] [CrossRef]
- Zimmerman, R.K.; Lin, C.J.; Raviotta, J.M.; Nowalk, M.P. Do vitamin D levels affect antibody titers produced in response to HPV vaccine? Hum. Vaccin. Immunother. 2015, 11, 2345–2349. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.M.; Herter-Aeberli, I.; Zimmermann, M.B.; Spieldenner, J.; Eggersdorfer, M. Strengthening the immunity of the Swiss population with micronutrients: A narrative review and call for action. Clin. Nutr. ESPEN 2021, 43, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Pecina, J.L.; Merry, S.P.; Park, J.G.; Thacher, T.D. Vitamin D Status and Severe COVID-19 Disease Outcomes in Hospitalized Patients. J. Prim. Care Community Health 2021, 12, 21501327211041206. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Cosentini, E.; Batori, G.; Marini, E.; Lombardini, R.; Gargaro, M.; Fallarino, F.; Scarponi, A.M.; et al. Prevalence of vitamin D deficiency and its prognostic impact on patients hospitalized with COVID-19. Nutrition 2021, 91–92, 111408. [Google Scholar] [CrossRef]
- Reis, B.Z.; Fernandes, A.L.; Sales, L.P.; Santos, M.D.; Dos Santos, C.C.; Pinto, A.J.; Goessler, K.F.; Franco, A.S.; Duran, C.S.C.; Silva, C.B.R.; et al. Influence of vitamin D status on hospital length of stay and prognosis in hospitalized patients with moderate to severe COVID-19: A multicenter prospective cohort study. Am. J. Clin. Nutr. 2021, 114, 598–604. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Dyer, A.H.; McCluskey, P.; O’Brien, K.; Dowds, J.; Laird, E.; Bannan, C.; Bourke, N.M.; Cheallaigh, C.N.; Byrne, D.G.; et al. Investigating the Relationship between Vitamin D and Persistent Symptoms Following SARS-CoV-2 Infection. Nutrients 2021, 13, 2430. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.A.; Drenos, F. No evidence that vitamin D is able to prevent or affect the severity of COVID-19 in individuals with European ancestry: A Mendeslian randomisation study of open data. BMJ Nutr. Prev. Health 2021, 4, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Butler-Laporte, G.; Nakanishi, T.; Mooser, V.; Morrison, D.R.; Abdullah, T.; Adeleye, O.; Mamlouk, N.; Kimchi, N.; Afrasiabi, Z.; Rezk, N.; et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: A Mendelian randomization study. PLoS Med. 2021, 18, e1003605. [Google Scholar] [CrossRef] [PubMed]





| Sampling 1 | Sampling 2 | Sampling 3 | Sampling 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Female | Male | Female | Male | Female | Male | Female | Male |
| n = 110 | n = 16 | n = 99 | n = 16 | n = 99 | n = 14 | n = 48 | n = 8 | |
| Age | 47 | 42 | 47 | 42 | 47 | 42 | 48 | 44 |
| Median (IQR) | (37, 55) | (36, 53) | (38, 55) | (36, 53) | (38, 55) | (36, 51) | (37, 55) | (40, 53) |
| SARS-CoV-2 IgG (AU/mL) | 0.6 | 0.6 | 27 | 18 | 417 | 375 | 42 | 34 |
| Median (IQR) | (0.0, 2.0) | (0.0, 2.5) | (15, 40) | (15, 50) | (155, 570) | (235, 512) | (24, 63) | (28, 56) |
| 25(OH)D (ng/mL) | 24 | 20 | 22 | 22 | 23 | 19 | 26 | 27 |
| Median (IQR) | (18, 30) | (12, 26) | (16, 30) | (11, 28) | (16, 30) | (11, 25) | (21, 32) | (21, 30) |
| Supplement Use * | ||||||||
| Yes, n (%) | 47 (43%) | 5 (31%) | 45 (45%) | 5 (31%) | 46 (46%) | 4 (39%) | 20 (42%) | 3 (38%) |
| No, n (%) | 63 (57%) | 11 (69%) | 54 (55%) | 11 (69%) | 53 (54%) | 10 (61%) | 28 (58%) | 5 (62%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chillon, T.S.; Demircan, K.; Heller, R.A.; Hirschbil-Bremer, I.M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relationship between Vitamin D Status and Antibody Response to COVID-19 mRNA Vaccination in Healthy Adults. Biomedicines 2021, 9, 1714. https://doi.org/10.3390/biomedicines9111714
Chillon TS, Demircan K, Heller RA, Hirschbil-Bremer IM, Diegmann J, Bachmann M, Moghaddam A, Schomburg L. Relationship between Vitamin D Status and Antibody Response to COVID-19 mRNA Vaccination in Healthy Adults. Biomedicines. 2021; 9(11):1714. https://doi.org/10.3390/biomedicines9111714
Chicago/Turabian StyleChillon, Thilo Samson, Kamil Demircan, Raban Arved Heller, Ines Maria Hirschbil-Bremer, Joachim Diegmann, Manuel Bachmann, Arash Moghaddam, and Lutz Schomburg. 2021. "Relationship between Vitamin D Status and Antibody Response to COVID-19 mRNA Vaccination in Healthy Adults" Biomedicines 9, no. 11: 1714. https://doi.org/10.3390/biomedicines9111714
APA StyleChillon, T. S., Demircan, K., Heller, R. A., Hirschbil-Bremer, I. M., Diegmann, J., Bachmann, M., Moghaddam, A., & Schomburg, L. (2021). Relationship between Vitamin D Status and Antibody Response to COVID-19 mRNA Vaccination in Healthy Adults. Biomedicines, 9(11), 1714. https://doi.org/10.3390/biomedicines9111714