Fluorescence Based on Surface Plasmon Coupled Emission for Ultrahigh Sensitivity Immunoassay of Cardiac Troponin I
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Plasmonic Chip Fabrication
2.3. Procedures for the Sandwich Immune Reaction on the Surface of SPCE Chip
2.4. Instrument and Apparatus for Detecting SPCE-Enhanced Fluorescence
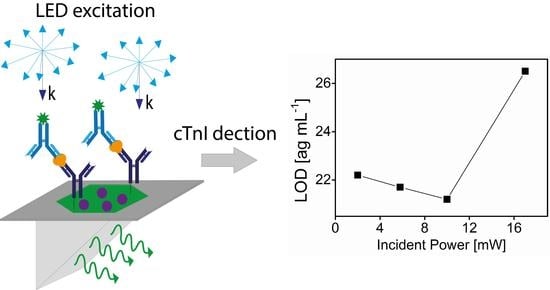
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kottwitz, J.; Bruno, K.A.; Berg, J.; Salomon, G.R.; Fairweather, D.; Elhassan, M.; Baltensperger, N.; Kissel, C.K.; Lovrinovic, M.; Baltensweiler, A.; et al. Myoglobin for Detection of High-Risk Patients with Acute Myocarditis. J. Cardiovasc. Transl. 2020, 13, 853–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matveeva, E.; Gryczynski, Z.; Gryczynski, I.; Malicka, J.; Lakowicz, J.R. Myoglobin Immunoassay Utilizing Directional Surface Plasmon-Coupled Emission. Anal. Chem. 2004, 76, 6287–6292. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Brent, J.R.; Shorie, M.; Kaur, H.; Chadha, G.; Thomas, A.G.; Lewis, E.A.; Rooney, A.P.; Nguyen, L.; Zhong, X.L.; et al. Nanostructured Aptamer-Functionalized Black Phosphorus Sensing Platform for Label-Free Detection of Myoglobin, a Cardiovascular Disease Biomarker. ACS Appl. Mater. Interfaces 2016, 8, 22860–22868. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Joung, H.-A.; Kim, M.-G.; Park, C.B. Graphene-Based Chemiluminescence Resonance Energy Transfer for Homogeneous Immunoassay. ACS Nano 2012, 6, 2978–2983. [Google Scholar] [CrossRef]
- Yeh, E.T. High-sensitivity C-reactive protein as a risk assessment tool for cardiovascular disease. Clin. Cardiol. 2006, 28, 408–412. [Google Scholar] [CrossRef]
- Grabowska, I.; Sharma, N.; Vasilescu, A.; Iancu, M.; Badea, G.; Boukherroub, R.; Ogale, S.; Szunerits, S. Electrochemical Aptamer-Based Biosensors for the Detection of Cardiac Biomarkers. ACS Omega 2018, 3, 12010–12018. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Han, Z.; Wang, X.; Li, F.; Cui, H.; Yang, D.; Bian, Z. Sensitive Immunosensor for N- Terminal Pro-brain Natriuretic Peptide Based on N-(Aminobutyl)-N-(ethylisoluminol)-Functionalized Gold Nanodots/Multiwalled Carbon Nanotube Electrochemiluminescence Nanointerface. Analyst 2015, 7, 7599–7604. [Google Scholar] [CrossRef]
- Takashio, S.; Yamamuro, M.; Izumiya, Y.; Hirakawa, K.; Marume, K.; Yamamoto, M.; Ueda, M.; Yamashita, T.; Ishibashi-Ueda, H.; Yasuda, S.; et al. Diagnostic utility of cardiac troponin T level in patients with cardiac amyloidosis. ESC Heart Fail. 2018, 5, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Song, K.-S.; Nimse, S.B.; Sonawane, M.D.; Lin, Y.; Zhou, Z.; Kim, T. A glass fibre membrane platform for ultra-sensitive detection of cardiac troponin T. Analyst 2017, 142, 3816–3821. [Google Scholar] [CrossRef]
- Zhou, W.; Li, K.; Wei, Y.; Hao, P.; Chi, M.; Liu, Y.; Wu, Y. Ultrasensitive label-free optical microfiber coupler biosensor for detection of cardiac Troponin I based on interference turning point effect. Biosens. Bioelectron. 2018, 106, 99–104. [Google Scholar] [CrossRef]
- Spain, E.; Carrara, S.; Adamson, K.; Ma, H.; Kennedy, R.O.; Cola, L.D.; Forster, R.J. Cardiac Troponin I: Ultrasensitive Detection Using Faradaic Electrochemical Impedance. ACS Omega 2018, 3, 17116–17124. [Google Scholar] [CrossRef]
- Fathil, M.F.M.; Arshad, M.K.M.; Gopinath, S.C.B.; Hashim, U.; Adzhri, R.; Ayub, R.M.; Ruslinda, A.R.; Nuzaihan, M.N.M.; Azman, A.H.; Zaki, M.; et al. Diagnostics on acute myocardial infarction: Cardiac troponin biomarkers. Biosens. Bioelectron. 2015, 70, 209–220. [Google Scholar] [CrossRef]
- Sabek, J.; Martínez-Pérez, P.; García-Rupérez, J. Computational binding study of cardiac Troponin I antibody towards cardiac versus skeletal Troponin I. Comput. Biol. Chem. 2019, 80, 147–151. [Google Scholar] [CrossRef]
- Nandhikonda, P.; Heagy, M.D. An Abiotic Fluorescent Probe for Cardiac Troponin I. J. Am. Chem. Soc. 2011, 133, 14972–14974. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Li, X.; Xu, Z.; Chen, L.; Li, D.; Dong, Y. Thionin functionalized signal amplification label derived dual-mode electrochemical immunoassay for sensitive detection of cardiac Troponin I. Biosens. Bioelectron. 2019, 133, 72–78. [Google Scholar] [CrossRef]
- Sun, D.; Luo, Z.; Lu, J.; Zhang, S.; Che, T.; Chen, Z.; Zhang, L. Electrochemical dual-aptamer-based biosensor for nonenzymatic detection of cardiac Troponin I by nanohybrid electrocatalysts labeling combined with DNA nanotetrahedron structure. Biosens. Bioelectron. 2019, 134, 49–56. [Google Scholar] [CrossRef]
- Fathil, M.F.M.; Arshad, M.K.M.; Ruslinda, A.R.; Gopinath, S.C.B.; Nuzaihan, M.N.M.; Adzhri, R.; Hashim, U.; Lam, H.Y. Substrate-gate coupling in ZnO-FET biosensor for cardiac Troponin I detection. Sens. Actuators B Chem. 2017, 242, 1142–1154. [Google Scholar] [CrossRef]
- Sarangadharan, I.; Regmi, A.; Chen, Y.-W.; Hsu, C.-P.; Chen, P.-C.; Chang, W.; Lee, G.-Y.; Chyi, J.-I.; Shiesh, S.-C.; Lee, G.-B.; et al. High sensitivity cardiac Troponin I detection in physiological environment using AlGaN/GaN High Electron Mobility Transistor (HEMT) Biosensors. Biosens. Bioelectron. 2018, 100, 282–289. [Google Scholar] [CrossRef]
- Gopinathan, P.; Sinha, A.; Chung, Y.-D.; Shiesh, S.-C.; Lee, G.-B. Optimization of an enzyme linked DNA aptamer assay for cardiac Troponin I detection: Synchronous multiple sample analysis on an integrated microfluidic platform. Analyst 2019, 144, 4943–4951. [Google Scholar] [CrossRef]
- Kar, P.; Pandey, A.; Greer, J.J.; Shankar, K. Ultrahigh sensitivity assays for human cardiac Troponin I using TiO2 nanotube arrays. Lab Chip 2012, 12, 821–828. [Google Scholar] [CrossRef]
- Miao, L.; Jiao, L.; Tang, Q.; Li, H.; Zhang, L.; Wei, Q. A nanozyme-linked immunosorbent assay for dual-modal colorimetric and ratiometric fluorescent detection of cardiac Troponin I. Sens. Actuators B Chem. 2019, 288, 60–64. [Google Scholar] [CrossRef]
- Kwon, Y.-C.; Kim, M.-G.; Kim, E.-M.; Shin, Y.-B.; Lee, S.-K.; Lee, S.D.; Cho, M.-J.; Ro, H.-S. Development of a surface plasmon resonance-based immunosensor for the rapid detection of cardiac Troponin I. Biotechnol. Lett. 2011, 33, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Çimen, D.; Bereli, N.; Günaydın, S.; Denizli, A. Detection of cardiac troponin-I by optic biosensors with immobilized anticardiac troponin-I monoclonal antibody. Talanta 2020, 219, 121259. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Jung, J.; Hahn, Y.K.; Kim, S.K.; Lee, Y.; Lee, J.; Lee, T.-H.; Park, J.-Y.; Seo, H.; Lee, J.N.; et al. A centrifugally actuated point-of-care testing system for the surface acoustic wave immunosensing of cardiac Troponin I. Analyst 2013, 138, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Mulpur, P.; Yadavilli, S.; Rao, A.M.; Kamisetti, V.; Podila, R. MoS2/WS2/BN-Silver Thin-Film Hybrid Architectures Displaying Enhanced Fluorescence via Surface Plasmon Coupled Emission for Sensing Applications. ACS Sens. 2016, 1, 826–833. [Google Scholar] [CrossRef]
- Tran, N.H.T.; Trinh, K.T.L.; Lee, J.-H.; Yoon, W.J.; Ju, H. Reproducible Enhancement of Fluorescence by Bimetal Mediated Surface Plasmon Coupled Emission for Highly Sensitive Quantitative Diagnosis of Double-Stranded DNA. Small 2018, 1801385. [Google Scholar] [CrossRef]
- Calander, N. Theory and Simulation of Surface Plasmon-Coupled Directional Emission from Fluorophores at Planar Structures. Anal. Chem. 2004, 76, 2168–2173. [Google Scholar] [CrossRef]
- Enderlein, J.; Ruckstuhl, T. The efficiency of surface-plasmon coupled emission for sensitive fluorescence detection. Opt. Express 2005, 13, 8855–8865. [Google Scholar] [CrossRef]
- Trnavsky, M.; Enderlein, J.; Ruckstuhl, T.; McDonagh, C.; MacCraith, B.D. Experimental and theoretical evaluation of surface plasmon-coupled emission for sensitive fluorescence detection. J. Biomed. Opt. 2008, 13, 054021. [Google Scholar] [CrossRef]
- Rangełowa-Jankowska, S.; Jankowski, D.; Bogdanowicz, R.; Grobelna, B.; Bojarski, P. Surface Plasmon-Coupled Emission of Rhodamine 110 Aggregates in a Silica Nanolayer. J. Phys. Chem. Lett. 2012, 3, 3626–3631. [Google Scholar] [CrossRef]
- Nu, T.T.V.; Tran, N.H.T.; Nam, E.; Nguyen, T.T.; Yoon, W.J.; Cho, S.; Kim, J.; Chang, K.-A.; Ju, H. Blood-based immunoassay of tau proteins for early diagnosis of Alzheimer’s disease using surface plasmon resonance fiber sensors. RSC Adv. 2018, 8, 7855–7862. [Google Scholar] [CrossRef] [Green Version]
- Tran, V.T.; Yoon, W.J.; Lee, J.-H.; Ju, H. DNA sequence-induced modulation of bimetallic surface plasmons in optical fibers for sub-ppq (parts-per-quadrillion) detection of mercury ions in water. J. Mater. Chem. A 2018, 6, 23894–23902. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Nguyen, T.T.; Lee, R.; Li, T.; Yun, C.; Ham, Y.; An, S.S.A.; Ju, H. Label-Free Quantitative Immunoassay of Fibrinogen in Alzheimer Disease Patient Plasma Using Fiber Optical Surface Plasmon Resonance. J. Electron. Mater. 2016, 45, 2354–2360. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bea, S.O.; Kim, D.M.; Yoon, W.J.; Park, J.-W.; An, S.S.A.; Ju, H. A regenerative label-free fiber optic sensor using surface plasmon resonance for clinical diagnosis of fibrinogen. Int. J. Nanomed. 2015, 10, 155–163. [Google Scholar]
- Ong, B.H.; Yuan, X.; Tan, Y.Y.; Irawan, R.; Fang, X.; Zhang, L.; Tjin, S.C. Two-layered metallic film-induced surface plasmon polariton for fluorescence emission enhancement in on-chip waveguide. Lab Chip 2007, 7, 506–512. [Google Scholar] [CrossRef]
- Mabe, T.; Zeng, Z.; Bagra, B.; Ryan, J.; Wei, J. Surface Plasmon Resonance of A Bimetallic Nanostructured Film for Enhanced Optical Sensitivity. ChemistrySelect 2018, 3, 3018–3023. [Google Scholar] [CrossRef]
- Wang, L.; Gaigalas, A.K.; Reipa, V. Optical properties of Alexa™ 488 and Cy™5 immobilized on a glass surface. Biotechniques 2005, 38, 127–132. [Google Scholar] [CrossRef]
- McPeak, K.M.; Jayanti, S.V.; Kress, S.J.P.; Meyer, S.; Iotti, S.; Rossinelli, A.; Norris, D.J. Plasmonic Films Can Easily Be Better: Rules and Recipes. ACS Photonics 2015, 2, 326–333. [Google Scholar] [CrossRef]
- Ciesielski, A.; Skowronski, L.; Trzcinski, M.; Górecka, E.; Trautman, P.; Szoplik, T. Evidence of germanium segregation in gold thin films. Surf. Sci. 2018, 674, 73–78. [Google Scholar] [CrossRef]
- Weber, W.H.; Eagen, C.F. Energy transfer from an excited dye molecule to the surface plasmons of an adjacent metal. Opt. Lett. 1979, 4, 236–238. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Wang, M.; Li, H.; Saqib, M.; Ge, C.; Zhang, X.; Jin, Y. Enhancing Luminol Electrochemiluminescence by Combined Use of Cobalt-Based Metal Organic Frameworks and Silver Nanoparticles and Its Application in Ultrasensitive Detection of Cardiac Troponin I. Anal. Chem. 2019, 91, 3048–3054. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Han, Q.; Chi, T.; Xing, B.; Ma, N.; Wu, D.; Wei, Q. Manganese doped CdS sensitized graphene/Cu2MoS4 composite for the photoelectrochemical immunoassay of cardiac Troponin I. Biosens. Bioelectron. 2019, 132, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lou, D.; Fan, L.; Cui, Y.; Zhu, Y.; Gu, N.; Zhang, Y. Fluorescent Nanoprobes with Oriented Modified Antibodies to Improve Lateral Flow Immunoassay of Cardiac Troponin I. Anal. Chem. 2018, 90, 6502–6508. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, Y.; Zhang, D.; Li, S.; Zhang, Y.; Ma, P.; Yu, Y.; Wang, X.; Song, D. Ultrasensitive magnetic field-assisted surface plasmon resonance immunoassay for human cardiac Troponin I. Biosens. Bioelectron. 2017, 96, 288–293. [Google Scholar] [CrossRef]
- Diware, M.S.; Cho, H.M.; Chegal, W.; Cho, Y.J.; Kim, D.S.; Kim, K.S.; Paek, S.H. Ultrasensitive, label-free detection of cardiac biomarkers with optical SIS sensor. Biosens. Bioelectron. 2017, 87, 242–248. [Google Scholar] [CrossRef]
- Baumberg, J.J.; Aizpurua, J.; Mikkelsen, M.H.; Smith, D.R. Extreme nanophotonics from ultrathin metallic gaps. Nat. Mater. 2019, 18, 668–678. [Google Scholar] [CrossRef]
- Muskens, O.L.; Giannini, V.; Sánchez-Gil, J.A.; Rivas, J.G. Strong Enhancement of the Radiative Decay Rate of Emitters by Single Plasmonic Nanoantennas. Nano Lett. 2007, 7, 2871–2875. [Google Scholar] [CrossRef]
- Gryczynski, I.; Malicka, J.; Nowaczyk, K.; Gryczynski, Z.; Lakowicz, J.R. Effects of Sample Thickness on the Optical Properties of Surface Plasmon-Coupled Emission. J. Phys. Chem. B 2004, 108, 12073–12083. [Google Scholar] [CrossRef] [Green Version]
- Badugu, R.; Szmacinski, H.; Ray, K.; Descrovi, E.; Ricciardi, S.; Zhang, D.; Chen, J.; Huo, Y.; Lakowicz, J.R. Metal-Dielectric Waveguides for High-Efficiency Coupled Emission. ACS Photonics 2015, 2, 810–815. [Google Scholar] [CrossRef] [Green Version]
- Gryczynski, I.; Malicka, J.; Nowaczyk, K.; Gryczynski, Z.; Lakowicz, J.R. Waveguide-Modulated Surface Plasmon-Coupled Emission of Nile Blue in Poly(Vinyl Alcohol) Thin Films. Thin Solid Films 2006, 510, 15–20. [Google Scholar] [CrossRef] [Green Version]



| cTnI Detection Methods | Concentration Range | LOD (with No Serum Used) | Ref. |
|---|---|---|---|
| Surface plasmon coupled emission (SPCE) | 0–0.5 pg mL−1 | 21.1 ag mL−1 | Present work |
| Optical microfiber coupler | 2–10 fg mL−1 | 2 fg mL−1 | [10] |
| Chemiluminescence | 0–1.5 ng mL−1 | 0.012 ng mL−1 | [19] |
| Electrochemiluminescence | 1 fg mL−1–1 μg mL−1 | 0.58 fg mL−1 | [41] |
| Photoelectrochemistry | 0.0005–1000 ng mL−1 | 0.18 pg mL−1 | [42] |
| Fluorescence | 0.05–32 ng mL−1 | 0.032 ng mL−1 | [43] |
| Magnetic field-assisted Surface plasmon resonance | 50–125 μg mL−1 | 1.25 ng mL−1 | [44] |
| Surface plasmon resonance | 0–0.160 μg mL−1 | 0.068 ng mL−1 | [22] |
| Optical solution immersed silicon | 0.005–10 ng mL−1 | 5 and 10 pg mL−1 | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, V.T.; Ju, H. Fluorescence Based on Surface Plasmon Coupled Emission for Ultrahigh Sensitivity Immunoassay of Cardiac Troponin I. Biomedicines 2021, 9, 448. https://doi.org/10.3390/biomedicines9050448
Tran VT, Ju H. Fluorescence Based on Surface Plasmon Coupled Emission for Ultrahigh Sensitivity Immunoassay of Cardiac Troponin I. Biomedicines. 2021; 9(5):448. https://doi.org/10.3390/biomedicines9050448
Chicago/Turabian StyleTran, Vien Thi, and Heongkyu Ju. 2021. "Fluorescence Based on Surface Plasmon Coupled Emission for Ultrahigh Sensitivity Immunoassay of Cardiac Troponin I" Biomedicines 9, no. 5: 448. https://doi.org/10.3390/biomedicines9050448
APA StyleTran, V. T., & Ju, H. (2021). Fluorescence Based on Surface Plasmon Coupled Emission for Ultrahigh Sensitivity Immunoassay of Cardiac Troponin I. Biomedicines, 9(5), 448. https://doi.org/10.3390/biomedicines9050448