Local Inhibition of Indoleamine 2,3-Dioxygenase Mitigates Renal Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals
2.3. Experimental Design and Surgical Procedures
2.4. Precision-Cut Kidney Slices (PCKS)
2.5. Real-Time Quantitative PCR
2.6. Western Blotting
2.7. Histology
2.8. Statistics
3. Results
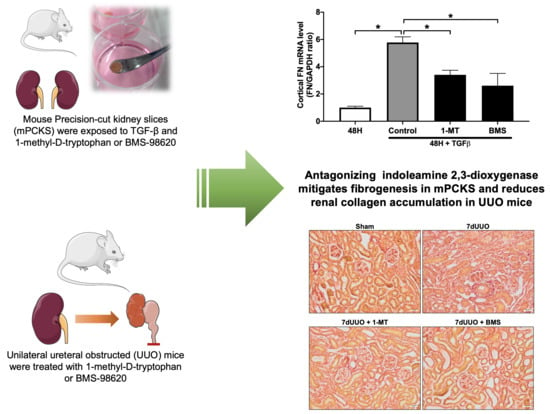
3.1. 1-MT Attenuates TGF-β-Induced Renal Fibrosis in mPCKS
3.2. BMS-986205 Mitigates TGF-β-Induced Fibrogenesis but Not Inflammation in mPCKS
3.3. 1-MT and BMS-986205 Treatment Attenuates Renal Collagen Deposition in UUO Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jha, V.; Garcia, G.G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef]
- Schefold, J.C.; Zeden, J.-P.; Fotopoulou, C.; Von Haehling, S.; Pschowski, R.; Hasper, D.; Volk, H.-D.; Schuett, C.; Reinke, P. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: A possible link between chronic inflammation and uraemic symptoms. Nephrol. Dial. Transplant. 2009, 24, 1901–1908. [Google Scholar] [CrossRef] [Green Version]
- Mutsaers, H.A.M.; Masereeuw, R.; Olinga, P. Altered tryptophan metabolism and CKD-associated fatigue. Kidney Int. 2014, 86, 1061–1062. [Google Scholar] [CrossRef] [Green Version]
- Stone, T.W.; Darlington, L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002, 1, 609–620. [Google Scholar] [CrossRef]
- Li, Y.; Kilani, R.T.; Rahmani-Neishaboor, E.; Jalili, R.B.; Ghahary, A. Kynurenine Increases Matrix Metalloproteinase-1 and -3 Expression in Cultured Dermal Fibroblasts and Improves Scarring In Vivo. J. Investig. Dermatol. 2014, 134, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Poormasjedi-Meibod, M.-S.; Hartwell, R.; Kilani, R.T.; Ghahary, A. Anti-Scarring Properties of Different Tryptophan Derivatives. PLoS ONE 2014, 9, e91955. [Google Scholar] [CrossRef]
- Darakhshan, S.; Pour, A.B. Tranilast: A review of its therapeutic applications. Pharmacol. Res. 2015, 91, 15–28. [Google Scholar] [CrossRef]
- Bao, Y.-S.; Ji, Y.; Zhao, S.-L.; Ma, L.-L.; Xie, R.-J.; Na, S.-P. Serum levels and activity of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA synthetase and their association with disease severity in patients with chronic kidney disease. Biomarkers 2013, 18, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Mohib, K.; Wang, S.; Guan, Q.; Mellor, A.L.; Sun, H.; Du, C.; Jevnikar, A.M. Indoleamine 2,3-dioxygenase expression promotes renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2008, 295, F226–F234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matheus, L.H.G.; Simão, G.M.; Amaral, T.A.; Brito, R.B.O.; Malta, C.S.; Matos, Y.S.T.; Santana, A.C.; Rodrigues, G.G.C.; Albejante, M.C.; Bach, E.E.; et al. Indoleamine 2,3-dioxygenase (IDO) increases during renal fibrogenesis and its inhibition potentiates TGF-β 1-induced epithelial to mesenchymal transition. BMC Nephrol. 2017, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Van den Brand, J.A.J.G.; Mutsaers, H.A.M.; Van Zuilen, A.D.; Blankestijn, P.J.; van den Broek, P.H.; Russel, F.G.M.; Masereeuw, R.; Wetzels, J.F.M. Uremic Solutes in Chronic Kidney Disease and Their Role in Progression. PLoS ONE 2016, 11, e0168117. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hasan, A.U.; Kobori, H. Melatonin in chronic kidney disease: A promising chronotherapy targeting the intrarenal renin–angiotensin system. Hypertens. Res. 2019, 42, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, H.A.M.; Wilmer, M.; Reijnders, D.; Jansen, J.; van den Broek, P.H.; Forkink, M.; Schepers, E.; Glorieux, G.; Vanholder, R.; van den Heuvel, L.P.; et al. Uremic toxins inhibit renal metabolic capacity through interference with glucuronidation and mitochondrial respiration. Biochim. Biophys. Acta 2013, 1832, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, H.A.M.; van den Heuvel, L.P.; Ringens, L.H.J.; Dankers, A.C.A.; Russel, F.; Wetzels, J.F.M.; Hoenderop, J.G.; Masereeuw, R. Uremic Toxins Inhibit Transport by Breast Cancer Resistance Protein and Multidrug Resistance Protein 4 at Clinically Relevant Concentrations. PLoS ONE 2011, 6, e18438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prendergast, G.C.; Malachowski, W.; DuHadaway, J.B.; Muller, A.J. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res. 2017, 77, 6795–6811. [Google Scholar] [CrossRef] [Green Version]
- Stribos, E.G.D.; Seelen, M.A.; Van Goor, H.; Olinga, P.; Mutsaers, H.A.M. Murine Precision-Cut Kidney Slices as an ex vivo Model to Evaluate the Role of Transforming Growth Factor-β1 Signaling in the Onset of Renal Fibrosis. Front. Physiol. 2017, 8, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gürtler, A.; Kunz, N.; Gomolka, M.; Hornhardt, S.; Friedl, A.; McDonald, K.; Kohn, J.E.; Posch, A. Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013, 433, 105–111. [Google Scholar] [CrossRef]
- Jensen, M.S.; Mutsaers, H.A.M.; Tingskov, S.J.; Christensen, M.; Madsen, M.G.; Olinga, P.; Kwon, T.H.; Nørregaard, R. Activation of the prostaglandin E2 EP2 receptor attenuates renal fibrosis in unilateral ureteral obstructed mice and human kidney slices. Acta Physiol 2019, 227, e13291. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.; Zhang, F.; Sun, J.; Chen, D.; Huang, W.; Zhang, H.; Cao, C.; Wan, X. Correlation of Indoleamine-2,3-Dioxygenase and Chronic Kidney Disease: A Pilot Study. J. Immunol. Res. 2021, 2021, 8132569. [Google Scholar] [CrossRef]
- Goek, O.-N.; Prehn, C.; Sekula, P.; Römisch-Margl, W.; Döring, A.; Gieger, C.; Heier, M.; Koenig, W.; Wang-Sattler, R.; Illig, T.; et al. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol. Dial. Transplant. 2013, 28, 2131–2138. [Google Scholar] [CrossRef] [Green Version]
- Debnath, S.; Velagapudi, C.; Redus, L.; Thameem, F.; Kasinath, B.; Hura, C.E.; Lorenzo, C.; Abboud, H.E.; O’Connor, J.C. Tryptophan Metabolism in Patients With Chronic Kidney Disease Secondary to Type 2 Diabetes: Relationship to Inflammatory Markers. Int. J. Tryptophan Res. 2017, 10, 1178646917694600. [Google Scholar] [CrossRef]
- Lee, H.; Jang, H.B.; Yoo, M.-G.; Park, S.I.; Lee, H.-J. Amino Acid Metabolites Associated with Chronic Kidney Disease: An Eight-Year Follow-Up Korean Epidemiology Study. Biomedicines 2020, 8, 222. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Benkowitz, P.; Lamina, C.; Köttgen, A.; Sekula, P. The relationship between blood metabolites of the tryptophan pathway and kidney function: A bidirectional Mendelian randomization analysis. Sci. Rep. 2020, 10, 12675. [Google Scholar] [CrossRef] [PubMed]
- Laurans, L.; Venteclef, N.; Haddad, Y.; Chajadine, M.; Alzaid, F.; Metghalchi, S.; Sovran, B.; Denis, R.; Dairou, J.; Cardellini, M.; et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat. Med. 2018, 24, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol. Sci. 2021, 42, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Zhu, R.; Jiang, T.; Tang, D.; Kwan, H.Y.; Su, T. Danshensu, a novel indoleamine 2,3-dioxygenase1 inhibitor, exerts anti-hepatic fibrosis effects via inhibition of JAK2-STAT3 signaling. Phytomedicine 2019, 63, 153055. [Google Scholar] [CrossRef]
- Zhong, W.; Gao, L.; Zhou, Z.; Lin, H.; Chen, C.; Huang, P.; Huang, W.; Zhou, C.; Huang, S.; Nie, L.; et al. Indoleamine 2,3-dioxygenase 1 deficiency attenuates CCl4-induced fibrosis through Th17 cells down-regulation and tryptophan 2,3-dioxygenase compensation. Oncotarget 2017, 8, 40486–40500. [Google Scholar] [CrossRef] [Green Version]
- Hoshi, M.; Osawa, Y.; Nakamoto, K.; Morita, N.; Yamamoto, Y.; Ando, T.; Tashita, C.; Nabeshima, T.; Saito, K. Kynurenine produced by indoleamine 2,3-dioxygenase 2 exacerbates acute liver injury by carbon tetrachloride in mice. Toxicology 2020, 438, 152458. [Google Scholar] [CrossRef]
- Ogiso, H.; Ito, H.; Ando, T.; Arioka, Y.; Kanbe, A.; Ando, K.; Ishikawa, T.; Saito, K.; Hara, A.; Moriwaki, H.; et al. The Deficiency of Indoleamine 2,3-Dioxygenase Aggravates the CCl4-Induced Liver Fibrosis in Mice. PLoS ONE 2016, 11, e0162183. [Google Scholar] [CrossRef] [Green Version]
- Milosavljevic, N.; Gazdic, M.; Markovic, B.S.; Arsenijevic, A.; Nurković, J.S.; Dolicanin, Z.; Jovicic, N.; Jeftic, I.; Djonov, V.; Arsenijevic, N.; et al. Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells—An experimental study. Transpl. Int. 2017, 31, 102–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-W.; Oh, J.E.; Rhee, K.-J.; Yoo, B.-S.; Eom, Y.W.; Park, S.W.; Lee, J.H.; Son, J.-W.; Youn, Y.J.; Ahn, M.-S.; et al. Co-treatment with interferon-γ and 1-methyl tryptophan ameliorates cardiac fibrosis through cardiac myofibroblasts apoptosis. Mol. Cell. Biochem. 2019, 458, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poosti, F.; Pham, B.T.; Oosterhuis, D.; Poelstra, K.; van Goor, H.; Olinga, P.; Hillebrands, J.-L. Precision-cut kidney slices (PCKS) to study development of renal fibrosis and efficacy of drug targeting ex vivo. Dis. Model. Mech. 2015, 8, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, F.; Teekamp, N.; Post, E.; Schuppan, D.; Kim, Y.; Zuidema, J.; Steendam, R.; Klose, M.H.; Meier-Menches, S.M.; Casini, A.; et al. The antifibrotic potential of a sustained release formulation of a PDGFβ-receptor targeted rho kinase inhibitor. J. Control. Release 2019, 296, 250–257. [Google Scholar] [CrossRef] [PubMed]






| Target Gene | Forward Primer | Reverse Primer |
|---|---|---|
| COL1 | 5′-CACCCTCAAGAGCCTGAGTC-3′ | 5′-ACTCTCCGCTCTTCCAGTCA-3′ |
| COL3 | 5′-GCACAGCAGTCCAACGTAGA-3′ | 5′-TCTCCAAATGGGATCTCTGG-3′ |
| FN | 5′-AATGGAAAAGGGGAATGGAC-3′ | 5′-CTCGGTTGTCCTTCTTGCTC-3′ |
| αSMA | 5′-CTGACAGAGGCACCACTGAA-3′ | 5′-CATCTCCAGAGTCCAGCACA-3′ |
| TNFα | 5′-AGGCTGCCCCGACTACGT-3′ | 5′-GACTTTCTCCTGGTATGAGATAGCAAA-3′ |
| IL-1β | 5′-CAGGCAGGCAGTATCACTCA-3′ | 5′-TGTCCTCATCCTGGAAGGTC-3′ |
| PAI-1 | 5′-AGTCTTTCCGACCAAGAGCA-3′ | 5′-GACAAAGGCTGTGGAGGAAG-3′ |
| GAPDH | 5´-TAAAGGGCATCCTGGGCTACACT-3′ | 5′-TTACTCCTTGGAGGCCATGTAGG-3′ |
| 18S | 5′-TGTGGTGTTGAGGAAAGCAG-3′ | 5′-TCCCATCCTTCACATCCTTC-3′ |
| Target | Catalog No. | Company | Dilution |
|---|---|---|---|
| FN | Ab2413 | Abcam | 1:2000 |
| αSMA | MO0851 | Dako | 1:500 |
| Smad2 | 3102 | Cell signaling | 1:2000 |
| pSmad2 | 3108 | Cell signaling | 1:500 |
| IDO1 | MAB5412 | Merck Millipore | 1:500 |
| Sham | Sham + 1-MT | 7dUUO | 7dUUO + 1-MT | |
|---|---|---|---|---|
| Bodyweight (g) | 21.7 ± 1.4 | 22.5 ± 1.9 | 21.8 ± 1.9 | 22.2 ± 1.4 |
| Left kidney/Bodyweight (mg/g) | 5.8 ± 0.7 | 5.7 ± 0.3 | 6.7 ± 0.6 * | 6.5 ± 0.3 * |
| PNa (mmol/L) | 146.8 ± 1.28 | 148 ± 1.58 | 147.4 ± 1.33 | 147.4 ± 1.43 |
| PK (mmol/L) | 4.5 ± 0.48 | 4.3 ± 0.14 | 4.4 ± 0.27 | 4.5 ± 0.42 |
| Creatinine (mmol/L) | 12.6 ± 5.32 | 18.0 ± 3.97 | 17.3 ± 3.35 * | 16.1 ± 4.44 |
| Osmol (mOsmol/kg) | 333.1 ± 18 | 337.8 ± 8.07 | 331 ± 9.31 | 329.3 ± 9.61 |
| BUN (mmol/L) | 4.9 ± 1.61 | 5.5 ± 1.46 | 6.6 ± 1.33 * | 6.5 ± 1 * |
| Sham | Sham + BMS | 7dUUO | 7dUUO + BMS | |
|---|---|---|---|---|
| Bodyweight (g) | 24.15 ± 1.9 | 23.15 ± 0.4 | 21.66 ± 1.3 * | 21.92 ± 1.4 * |
| Left kidney/Bodyweight (mg/g) | 5.8 ± 0.7 | 5.9 ± 0.3 | 6.4 ± 0.5 | 6.8 ± 0.8 * |
| PNa (mmol/L) | 150 ± 1.63 | 149.8 ± 0.84 | 150.8 ± 0.45 | 150.4 ± 3.10 |
| PK (mmol/L) | 4.4 ± 0.50 | 4.2 ± 0.24 | 5.0 ± 0.72 | 4.7 ± 0.63 |
| Creatinine (mmol/L) | 13.3 ± 4.82 | 10.2 ± 4.05 | 14.9 ± 3.18 | 12.1 ± 2.65 |
| Osmol (mOsmol/kg) | 313.8 ± 2.22 | 311 ± 4 | 321.6 ± 14.77 | 323.5 ± 14.88 |
| BUN (mmol/L) | 5.8 ± 1.02 | 5.1 ± 1.05 | 7.3 ± 0.81* | 6.3 ± 1.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensen, C.G.; Jensen, M.S.; Tingskov, S.J.; Olinga, P.; Nørregaard, R.; Mutsaers, H.A.M. Local Inhibition of Indoleamine 2,3-Dioxygenase Mitigates Renal Fibrosis. Biomedicines 2021, 9, 856. https://doi.org/10.3390/biomedicines9080856
Jensen CG, Jensen MS, Tingskov SJ, Olinga P, Nørregaard R, Mutsaers HAM. Local Inhibition of Indoleamine 2,3-Dioxygenase Mitigates Renal Fibrosis. Biomedicines. 2021; 9(8):856. https://doi.org/10.3390/biomedicines9080856
Chicago/Turabian StyleJensen, Camilla Grønkjær, Michael Schou Jensen, Stine Julie Tingskov, Peter Olinga, Rikke Nørregaard, and Henricus A. M. Mutsaers. 2021. "Local Inhibition of Indoleamine 2,3-Dioxygenase Mitigates Renal Fibrosis" Biomedicines 9, no. 8: 856. https://doi.org/10.3390/biomedicines9080856
APA StyleJensen, C. G., Jensen, M. S., Tingskov, S. J., Olinga, P., Nørregaard, R., & Mutsaers, H. A. M. (2021). Local Inhibition of Indoleamine 2,3-Dioxygenase Mitigates Renal Fibrosis. Biomedicines, 9(8), 856. https://doi.org/10.3390/biomedicines9080856