Feasibility of Home-Based Pulmonary Rehabilitation of Pediatric Patients with Chronic Respiratory Diseases
Abstract
:1. Introduction
2. Methods
2.1. Participants
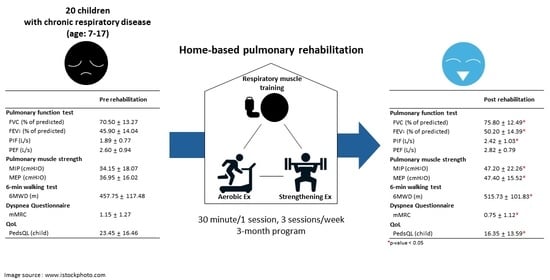
2.2. Home-Based Pulmonary Rehabilitation Program
2.3. Measurements
2.3.1. Pulmonary Function Test (PFT)
2.3.2. Respiratory Muscle Strength
2.3.3. Cardiopulmonary Exercise Test (CPET)
2.3.4. 6 Min Walk Test (6MWT)
2.3.5. Severity of Dyspnea
2.3.6. Quality of Life
2.3.7. Speech Evaluation
2.3.8. Feasibility Measure
2.3.9. Other Clinical Variables
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Compliance and Satisfaction of the Program
3.3. Effects of Home-Based Pulmonary Rehabilitation
3.4. Effect of Home-Based Pulmonary Rehabilitation According to Compliance
3.5. Effect of Home-Based Pulmonary Rehabilitation according to the Severity of the Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillerman, R.P.; Brody, A.S. Contemporary perspectives on pediatric diffuse lung disease. Radiol. Clin. N. Am. 2011, 49, 847–868. [Google Scholar] [CrossRef] [PubMed]
- Kabra, S.K.; Lodha, R.; Singhal, T. Chronic obstructive pulmonary disease in children. Indian J. Pediatr. 2001, 68 (Suppl. 2), S50–S54. [Google Scholar]
- Deolmi, M.; Decarolis, N.M.; Motta, M.; Makrinioti, H.; Fainardi, V.; Pisi, G.; Esposito, S. Early Origins of Chronic Obstructive Pulmonary Disease: Prenatal and Early Life Risk Factors. Int. J. Environ. Res. Public Health 2023, 20, 2294. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Wang, Y.; Lin, R.; Zeng, Y.; Chen, C.; Yang, L.; Yue, M.; Zhong, S.; Wang, Y.; Zhang, Q. Impact of early life exposures on COPD in adulthood: A systematic review and meta-analysis. Respirology 2021, 26, 1131–1151. [Google Scholar] [CrossRef]
- Baraldi, E.; Filippone, M. Chronic lung disease after premature birth. N. Engl. J. Med. 2007, 357, 1946–1955. [Google Scholar] [CrossRef]
- Counil, F.P.; Varray, A.; Matecki, S.; Beurey, A.; Marchal, P.; Voisin, M.; Préfaut, C. Training of aerobic and anaerobic fitness in children with asthma. J. Pediatr. 2003, 142, 179–184. [Google Scholar] [CrossRef]
- Ahmaidi, S.B.; Varray, A.L.; Savy-Pacaux, A.M.; Prefaut, C.G. Cardiorespiratory fitness evaluation by the shuttle test in asthmatic subjects during aerobic training. Chest 1993, 103, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Basaran, S.; Guler-Uysal, F.; Ergen, N.; Seydaoglu, G.; Bingol-Karakoç, G.; Ufuk Altintas, D. Effects of physical exercise on quality of life, exercise capacity and pulmonary function in children with asthma. J. Rehabil. Med. 2006, 38, 130–135. [Google Scholar] [CrossRef]
- Fanelli, A.; Cabral, A.L.; Neder, J.A.; Martins, M.A.; Carvalho, C.R. Exercise training on disease control and quality of life in asthmatic children. Med. Sci. Sports Exerc. 2007, 39, 1474–1480. [Google Scholar] [CrossRef]
- Núñez, I.R.; Araos, D.Z.; Delgado, C.M. Effects of home-based respiratory muscle training in children and adolescents with chronic lung disease. J. Bras. Pneumol. 2014, 40, 626–633. [Google Scholar] [CrossRef]
- Simpson, K.; Killian, K.; McCartney, N.; Stubbing, D.G.; Jones, N.L. Randomised controlled trial of weightlifting exercise in patients with chronic airflow limitation. Thorax 1992, 47, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Marquis, K.; Debigaré, R.; Lacasse, Y.; LeBlanc, P.; Jobin, J.; Carrier, G.; Maltais, F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 2002, 166, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.S.H.; Pitzner-Fabricius, A.; Toennesen, L.L.; Rasmusen, H.K.; Hostrup, M.; Hellsten, Y.; Backer, V.; Henriksen, M. Effect of aerobic exercise training on asthma in adults: A systematic review and meta-analysis. Eur. Respir. J. 2020, 56, 2000146. [Google Scholar] [CrossRef] [PubMed]
- Consensus Document on Pulmonary Rehabilitation in Korea 2015. Korean J. Intern. Med. Search 2015. Available online: https://www.lungkorea.org/bbs/index.html?code=guide&category=&gubun=&page=2&number=3484mode=view&keyfield=&key= (accessed on 24 October 2016).
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate; a longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar] [PubMed]
- Bhammar, D.M.; Jones, H.N.; Lang, J.E. Inspiratory Muscle Rehabilitation Training in Pediatrics: What Is the Evidence? Can. Respir. J. 2022, 2022, 5680311. [Google Scholar] [CrossRef] [PubMed]
- Crapo, R.O.; Casaburi, R.; Coates, A.L.; Enright, P.L.; Hankinson, J.L.; Irvin, C.G.; MacIntyre, N.R.; McKay, R.T.; Wanger, J.S.; Anderson, S.D.; et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am. J. Respir. Crit. Care. Med. 2000, 161, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Dafoe, W. Principles of exercise testing and interpretation. Can. J. Cardiol. 2007, 23, 274. [Google Scholar]
- Jeong, D.; Oh, Y.M.; Lee, S.W.; Lee, S.D.; Lee, J.S. Comparison of Predicted Exercise Capacity Equations in Adult Korean Subjects. J Korean Med. Sci 2022, 37, e113. [Google Scholar] [CrossRef]
- ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care. Med. 2002, 166, 111–117. [CrossRef]
- Fletcher, C.M.; Elmes, P.C.; Fairbairn, A.S.; Wood, C.H. Significance of Respiratory Symptoms and the Diagnosis of Chronic Bronchitis in a Working Population. Br. Med. J. 1959, 2, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Reddy, R.C.; Baptist, A.P. Pediatric Dyspnea Scale for use in hospitalized patients with asthma. J. Allergy Clin. Immunol. 2009, 123, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, E.; Clini, E.M. Measures of dyspnea in pulmonary rehabilitation. Multidiscip. Respir. Med. 2010, 5, 202. [Google Scholar] [CrossRef] [PubMed]
- Varni, J.W.; Seid, M.; Kurtin, P.S. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med. Care. 2001, 39, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Ryan, W.J.; Burk, K.W. Perceptual and acoustic correlates of aging in the speech of males. J. Commun. Disord. 1974, 7, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Pyo, H.Y. A comparison study of the characteristics of pauses and breath groups during paragraph reading for normal female adults with and without voice disorders. Phon. Speech Sci. 2019, 11, 109–116. [Google Scholar] [CrossRef]
- Park, S.S.; Choi, S.H.; Hong, J.A.; Hong, Y.H.; Jeong, N.G.; Lee, S.Y.; Sung, M.W.; Hah, J.H. Validity and reliability of the Korean version of the Speech Handicap Index in patients with oral cavity cancer. Int. J. Oral Maxillofac. Surg. 2016, 45, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Majd, S.; Apps, L.; Chantrell, S.; Hudson, N.; Eglington, E.; Hargadon, B.; Murphy, A.; Singh, S.J.; Bradding, P.; Green, R.H.; et al. A Feasibility Study of a Randomized Controlled Trial of Asthma-Tailored Pulmonary Rehabilitation Compared with Usual Care in Adults with Severe Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3418–3427. [Google Scholar] [CrossRef] [PubMed]
- Bonini, M.; Di Paolo, M.; Bagnasco, D.; Baiardini, I.; Braido, F.; Caminati, M.; Carpagnano, E.; Contoli, M.; Corsico, A.; Del Giacco, S.; et al. Minimal clinically important difference for asthma endpoints: An expert consensus report. Eur. Respir. Rev. 2020, 29, 190137. [Google Scholar] [CrossRef]
- Liddell, F.; Webber, J. Pulmonary rehabilitation for chronic obstructive pulmonary disease: A pilot study evaluating a once-weekly versus twice-weekly supervised programme. Physiotherapy 2010, 96, 68–74. [Google Scholar] [CrossRef]
- Lu, K.D.; Forno, E. Exercise and lifestyle changes in pediatric asthma. Curr. Opin. Pulm. Med. 2020, 26, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Oates, G.R.; Niranjan, S.J.; Ott, C.; Scarinci, I.C.; Schumann, C.; Parekh, T.; Dransfield, M.T. Adherence to Pulmonary Rehabilitation in Copd: A qualitative exploration of patient perspectives on barriers and facilitators. J. Cardiopulm. Rehabil. Prev. 2019, 39, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Hayton, C.; Clark, A.; Olive, S.; Browne, P.; Galey, P.; Knights, E.; Staunton, L.; Jones, A.; Coombes, E.; Wilson, A.M. Barriers to pulmonary rehabilitation: Characteristics that predict patient attendance and adherence. Respir. Med. 2013, 107, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sooriyakanthan, M.; Orme, M.W.; Sivapalan, K.; Selvaratnam, G.; Singh, S.J.; Wimalasekera, S. A feasibility trial of pulmonary rehabilitation for patients with COPD in a low resource setting: Jaffna, Sri Lanka. BMC Pulm. Med. 2022, 22, 302. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, E.; Fulgenzi, A.; Ferrero, M.E. Rationale of the Combined Use of Inspiratory and Expiratory Devices in Improving Maximal Inspiratory Pressure and Maximal Expiratory Pressure of Patients With Chronic Obstructive Pulmonary Disease. Arch. Phys. Med. Rehabil. 2009, 90, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Silva, I.; Silva, T.E.; Fernandes, T.; Filho, N.L.; Oliveira, V.H.; Pedrosa, R.; Ferreira, G. Inspiratory muscle training for asthma—A randomized controlled pilot study. Eur. Respir. J. 2014, 44, P3358. [Google Scholar]
- Wang, Y.-T.; Green, J.R.; Nip, I.S.B.; Kent, R.D.; Kent, J.F.; Ullman, C. Accuracy of perceptually based and acoustically based inspiratory loci in reading. Behav. Res. Methods 2010, 42, 791–797. [Google Scholar] [CrossRef]
| Characteristics | |
|---|---|
| Age, mean (range) | 11.2 ± 3.1 (7–17) |
| Sex (male/female) | 10 (50%)/10 (50%) |
| Height (cm) | 144.1 ± 16.2 |
| Weight (kg) | 37.5 ± 14.2 |
| Diagnosis | |
| Bronchiolitis obliterans | 12 (60%) |
| Bronchopulmonary dysplasia | 5 (25%) |
| ETC | 3 (15%) |
| Time since diagnosis (years) | 7.7 ± 3.7 |
| Severity | |
| FEV1 ≥ 80% | 0 (0%) |
| 80% > FEV1 ≥ 60% | 3 (15%) |
| FEV1 < 60% | 17 (85%) |
| Type | |
| Obstructive | 15 (75%) |
| Restrictive | 2 (10%) |
| Mixed | 3 (15%) |
| Maintenance therapy | |
| Prn SABA | 12 (60%) |
| Daily ICS | 3 (15%) |
| Daily ICS/LAMA | 3 (15%) |
| Other (mixed therapy) | 2 (10%) |
| Total | 17.1 ± 5.0 |
| Video program satisfaction | |
| Convenience | 1.8 ± 0.7 |
| Interesting | 2.0 ± 0.9 |
| Image and design | 2.0 ± 0.8 |
| Sound and song | 1.8 ± 0.8 |
| Helpfulness | 1.8 ± 0.7 |
| Changes of daily life after participating in the intervention | |
| Feel my breathing improving | 1.9 ± 0.6 |
| More interested in respiratory health | 1.7 ± 0.8 |
| Do more walking exercises | 2.3 ± 0.8 |
| Do more breathing exercises | 2.1 ± 0.5 |
| Pre-Rehab | Post-Rehab | p-Value | |
|---|---|---|---|
| Pulmonary function test | |||
| FVC (L/s) | 1.85 ± 0.58 (70.50 ± 13.27%) | 2.01 ± 0.60 (75.80 ± 12.49%) | 0.001 |
| FEV1 (L/s) | 1.07 ± 0.36 (45.90 ± 14.04%) | 1.17 ± 0.38 (50.20 ± 14.39%) | <0.001 |
| FEV1/FVC (%) | 61.00 ± 17.67 | 60.15 ± 15.93 | 0.513 |
| PIF (L/s) | 1.89 ± 0.77 | 2.42 ± 1.03 | 0.045 |
| PEF (L/s) | 2.60 ± 0.94 | 2.82 ± 0.79 | 0.020 |
| Pulmonary muscle strength | |||
| MIP (cmH2O) | 34.15 ± 18.07 | 47.20 ± 22.26 | <0.001 |
| MEP (cmH2O) | 36.95 ± 16.02 | 47.40 ± 15.52 | <0.001 |
| CPET | |||
| VO2max | 19.85 ± 5.34 | 22.09 ± 8.31 | 0.134 |
| METs | 5.71 ± 1.49 | 6.31 ± 2.38 | 0.160 |
| RQ | 1.14 ± 0.12 | 1.24 ± 0.14 | 0.108 |
| 6 min walking test | |||
| 6MWD (m) | 457.75 ± 117.48 | 515.731 ± 101.83 | 0.003 |
| 6MWT Borg scale | 11.20 ± 3.14 | 12.20 ± 3.83 | 0.176 |
| Dyspnea Questionnaire | |||
| mMRC | 1.15 ± 1.27 | 0.75 ± 1.12 | 0.038 |
| PDS | 2.00 ± 1.08 | 1.65 ± 0.99 | 0.109 |
| OCD | 74.54 ± 11.79 | 77.25 ± 12.82 | 0.004 |
| QoL | |||
| PedsQL (child) | 23.45 ± 16.46 | 16.35 ± 13.59 | 0.004 |
| PedsQL (parent) | 25.10 ± 19.18 | 22.60 ± 18.80 | 0.456 |
| Speech evaluation | |||
| Maximal phonation time (s) | 7.68 ± 2.28 | 8.91 ± 1.91 | 0.069 |
| Speech rate (s) | 104.75 ± 31.11 | 89.50 ± 20.73 | 0.021 |
| Speech handicap index | 14.25 ± 13.56 | 12.38 ± 12.87 | 0.266 |
| Compliance ≥ 50% (n = 15) | Compliance < 50% (n = 5) | ||||||
|---|---|---|---|---|---|---|---|
| Pre-Rehab | Post-Rehab | p-Value | Pre-Rehab | Post-Rehab | p-Value | p-Value between the Group Differences | |
| Pulmonary function test | |||||||
| FVC (L/s) | 1.78 ± 0.53 (67.40 ± 12.40%) | 1.96 ± 0.55 (75.00 ± 13.28%) | 0.005 | 2.05 ± 0.76 (79. 80 ± 12.40%) | 2.13 ± 0.77 (78.20 ± 10.66%) | 0.416 | 0.026 |
| FEV1 (L/s) | 1.07 ± 0.31 (45.40 ± 13.55%) | 1.19 ± 0.55 (51.07 ± 14.08%) | 0.001 | 1.08 ± 0.53 (47.40 ± 17.02%) | 1.13 ± 0.52 (47.60 ± 16.68%) | 0.785 | 0.011 |
| FEV1/FVC (%) | 62.93 ± 16.29 | 61.47 ± 14.56 | 0.391 | 55.20 ± 22.31 | 56.20 ± 20.91 | 0.269 | 0.379 |
| PIF (L/s) | 1.72 ± 0.64 | 2.47 ± 0.94 | 0.001 | 2.42 ± 0.95 | 2.26 ± 1.38 | 0.686 | 0.040 |
| PEF (L/s) | 2.52 ± 0.97 | 2.86 ± 0.80 | 0.014 | 2.83 ± 0.91 | 2.71 ± 0.85 | 0.345 | 0.029 |
| Pulmonary muscle strength | |||||||
| MIP (cmH2O) | 33.27 ± 19.12 | 45.87 ± 23.66 | <0.001 | 36.80 ± 12.87 | 51.20 ± 19.18 | 0.078 | 0.631 |
| MEP (cmH2O) | 37.00 ± 17.35 | 47.13 ± 16.17 | 0.002 | 36.80 ± 12.87 | 48.20 ± 15.09 | 0.043 | >0.999 |
| CPET | |||||||
| VO2max | 21.25 ± 4.20 | 23.69 ± 7.41 | 0.219 | 15.20 ± 7.05 | 17.30 ± 10.69 | 0.285 | 0.964 |
| METs | 6.10 ± 1.18 | 6.77 ± 2.13 | 0.239 | 4.40 ± 1.93 | 4.93 ± 3.04 | 0.593 | 0.964 |
| RQ | 1.14 ± 0.13 | 1.28 ± 0.14 | 0.077 | 1.14 ± 0.07 | 1.11 ± 0.07 | 0.109 | 0.115 |
| 6 min walking test | |||||||
| 6MWD (m) | 455.70 ± 135.62 | 519.53 ± 108.77 | 0.008 | 463.90 ± 33.45 | 504.30 ± 87.36 | 0.225 | 0.513 |
| 6MWT Borg scale | 11.53 ± 3.20 | 12.13 ± 3.52 | 0.454 | 10.20 ± 3.03 | 12.40 ± 5.13 | 0.197 | 0.329 |
| Dyspnea questionnaire | |||||||
| mMRC | 1.33 ± 1.35 | 0.80 ± 1.21 | 0.038 | 0.60 ± 0.89 | 0.60 ± 0.89 | >0.999 | 0.150 |
| PDS | 2.13 ± 1.19 | 1.67 ± 1.05 | 0.058 | 1.60 ± 0.55 | 1.60 ± 0.89 | >0.999 | 0.425 |
| OCD | 69.97 ± 17.77 | 77.67 ± 13.21 | 0.008 | 74.00 ± 11.40 | 76.00 ± 12.94 | 0.157 | 0.214 |
| QoL | |||||||
| PedsQL (child) | 22.00 ± 18.03 | 12.73 ± 11.74 | 0.003 | 27.80 ± 10.83 | 27.20 ± 14.10 | 0.854 | 0.072 |
| PedsQL (parent) | 25.73 ± 21.13 | 19.53 ± 19.48 | 0.116 | 23.20 ± 13.37 | 31.80 ± 14.46 | 0.345 | 0.066 |
| FEV1 < 60% Group (n = 17) | FEV1 ≥ 60% Group (n = 3) | ||||||
|---|---|---|---|---|---|---|---|
| Pre-Rehab | Post-Rehab | p-Value | Pre-Rehab | Post-Rehab | p-Value | p-Value between the Group Differences | |
| Pulmonary function test | |||||||
| FVC (L/s) | 1.91 ± 0.47 (70.24 ± 14.03%) | 2.07 ± 0.51 (75.24 ± 13.04%) | 0.028 | 1.50 ± 1.13 (72.00 ± 9.64%) | 1.65 ± 1.06 (79.00 ± 10.15%) | 0.285 | 0.750 |
| FEV1(L/s) | 1.05 ± 0.30 (42.59 ± 12.42%) | 1.17 ± 0.33 (47.59 ± 13.96%) | 0.001 | 1.15 ± 0.72 (64.67 ± 4.04%) | 1.20 ± 0.69 (65.00 ± 4.58%) | 0.785 | 0.110 |
| FEV1/FVC (%) | 57.29 ± 16.14 | 57.53 ± 15.59 | 0.799 | 82.00 ± 10.15 | 75.00 ± 8.72 | 0.285 | 0.311 |
| PIF(L/s) | 1.86 ± 0.74 | 2.52 ± 1.04 | 0.010 | 2.06 ± 1.09 | 1.83 ± 0.93 | 0.285 | 0.050 |
| PEF (L/s) | 2.58 ± 0.92 | 2.84 ± 0.77 | 0.051 | 2.69 ± 1.29 | 2.74 ± 1.13 | >0.999 | 0.397 |
| Pulmonary muscle strength | |||||||
| MIP (cmH2O) | 35.94 ± 18.78 | 50.65 ± 21.83 | <0.001 | 24.00 ± 9.85 | 27.67 ± 14.84 | 0.593 | 0.100 |
| MEP (cmH2O) | 38.00 ± 16.83 | 48.47 ± 16.64 | 0.001 | 31.00 ± 10.54 | 41.33 ± 2.52 | 0.109 | 0.916 |
| CPET | |||||||
| VO2max | 19.58 ± 5.48 | 21.44 ± 8.40 | 0.212 | 23.20 ± 0.00 | 29.20 ± 0.00 | NA | 0.765 |
| METs | 5.63 ± 1.53 | 6.13 ± 2.41 | 0.247 | 6.60 ± 0.00 | 8.30 ± 0.00 | NA | 0.765 |
| RQ | 1.14 ± 0.12 | 1.25 ± 0.14 | 0.087 | 1.15 ± 0.00 | 1.09 ± 0.00 | NA | 0.479 |
| 6 min walking test | |||||||
| 6MWD (m) | 453.44 ± 127.22 | 505.91 ± 107.47 | 0.017 | 482.17 ± 24.17 | 571.33 ± 25.66 | 0.109 | 0.491 |
| 6MWT Borg scale | 11.71 ± 3.04 | 12.47 ± 3.50 | 0.307 | 8.33 ± 2.31 | 10.67 ± 6.11 | 0.276 | 0.451 |
| Dyspnea Questionnaire | |||||||
| mMRC | 1.35 ± 1.27 | 0.88 ± 1.17 | 0.038 | 0.00 ± 0.00 | 0.00 ± 0.00 | >0.999 | 0.295 |
| PDS | 2.12 ± 1.11 | 1.77 ± 1.03 | 0.166 | 1.33 ± 0.58 | 1.00 ± 0.00 | 0.317 | 0.955 |
| OCD | 68.79 ± 16.48 | 75.59 ± 13.10 | 0.008 | 83.33 ± 7.61 | 86.67 ± 5.77 | 0.157 | 0.667 |
| QoL | |||||||
| PedsQL (child) | 24.53 ± 17.67 | 16.71 ± 14.74 | 0.006 | 17.33 ± 3.06 | 14.33 ± 3.21 | 0.180 | 0.633 |
| PedsQL (parent) | 26.12 ± 20.08 | 23.47 ± 20.20 | 0.518 | 19.33 ± 14.67 | 17.67 ± 7.02 | 0.593 | 0.832 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.Y.; Mo, Y.H.; Kim, K.W.; Hong, S.M.; Park, A.; Jang, B.H.; Lee, S.H.; Lee, J.H.; Yoon, J.; Yu, J.; et al. Feasibility of Home-Based Pulmonary Rehabilitation of Pediatric Patients with Chronic Respiratory Diseases. Children 2024, 11, 534. https://doi.org/10.3390/children11050534
Kim DY, Mo YH, Kim KW, Hong SM, Park A, Jang BH, Lee SH, Lee JH, Yoon J, Yu J, et al. Feasibility of Home-Based Pulmonary Rehabilitation of Pediatric Patients with Chronic Respiratory Diseases. Children. 2024; 11(5):534. https://doi.org/10.3390/children11050534
Chicago/Turabian StyleKim, Da Yeong, Young Hoon Mo, Kun Woo Kim, Sae Mi Hong, Arum Park, Baek Hee Jang, Seung Hak Lee, Joon Hee Lee, Jisun Yoon, Jinho Yu, and et al. 2024. "Feasibility of Home-Based Pulmonary Rehabilitation of Pediatric Patients with Chronic Respiratory Diseases" Children 11, no. 5: 534. https://doi.org/10.3390/children11050534
APA StyleKim, D. Y., Mo, Y. H., Kim, K. W., Hong, S. M., Park, A., Jang, B. H., Lee, S. H., Lee, J. H., Yoon, J., Yu, J., & Ko, E. J. (2024). Feasibility of Home-Based Pulmonary Rehabilitation of Pediatric Patients with Chronic Respiratory Diseases. Children, 11(5), 534. https://doi.org/10.3390/children11050534