Can Anthropometry and Body Composition Explain Physical Fitness Levels in School-Aged Children?
Abstract
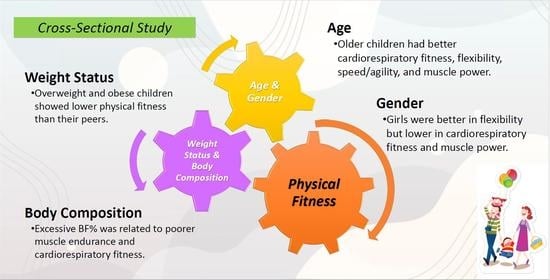
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Anthropometric Measures
2.3. Body Composition Parameters
2.4. Physical Fitness Levels
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Overall Cohort and Two Subgroups Stratified by Sex
3.2. Associations of Physical Fitness Levels and Variables of Interest in the Overall Cohort
3.3. Variables Independently Associated with Physical Fitness Levels in the Overall Cohort Using Logistic Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corbin, C.B.; Lindsey, R. Concepts of Physical Fitness; McGraw-Hill Education: New York, NY, USA, 1997. [Google Scholar]
- Pate, R.R. The Evolving Definition of Physical Fitness. Quest 1988, 40, 174–179. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Lee, P.-F.; Lee, T.-S.; Ho, C.-C. Poor Physical Fitness Performance as a Predictor of General Adiposity in Taiwanese Adults. Int. J. Environ. Res. Public Health 2020, 17, 2686. [Google Scholar] [CrossRef] [Green Version]
- Booth, F.W.; Roberts, C.K.; Laye, M. Lack of Exercise Is a Major Cause of Chronic Diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.B.; Ruiz, J.; Castillo, M.J.; Sjöström, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 2007, 32, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Henriques-Neto, D.; Magalhães, J.P.; Hetherington-Rauth, M.; Santos, D.A.; Baptista, F.; Sardinha, L. Physical Fitness and Bone Health in Young Athletes and Nonathletes. Sports Health 2020, 12, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Mäestu, E.; Harro, J.; Veidebaum, T.; Kurrikoff, T.; Jürimäe, J.; Mäestu, J. Changes in cardiorespiratory fitness through adolescence predict metabolic syndrome in young adults. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 701–708. [Google Scholar] [CrossRef]
- Åvitsland, A.; Leibinger, E.; Haugen, T.; Lerum, Ø.; Solberg, R.B.; Kolle, E.; Dyrstad, S.M. The association between physical fitness and mental health in Norwegian adolescents. BMC Public Health 2020, 20, 776. [Google Scholar] [CrossRef] [PubMed]
- Appelqvist-Schmidlechner, K.; Vaara, J.P.; Vasankari, T.; Häkkinen, A.; Mäntysaari, M.; Kyröläinen, H. Muscular and cardiorespiratory fitness are associated with health-related quality of life among young adult men. BMC Public Health 2020, 20, 842. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Ku, M.; Kiyoji, T.; Isobe, T.; Sakae, T.; Oh, S. Cardiorespiratory fitness is strongly linked to metabolic syndrome among physical fitness components: A retrospective cross-sectional study. J. Physiol. Anthr. 2020, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; Cheng, Y.; Gu, L.; Xie, Z. Cardiorespiratory fitness as a quantitative predictor of the risk of stroke: A dose–response meta-analysis. J. Neurol. 2020, 267, 491–501. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.-C.; Martínez-Vizcaíno, V. Muscular Strength as a Predictor of All-Cause Mortality in an Apparently Healthy Population: A Systematic Review and Meta-Analysis of Data from Approximately 2 Million Men and Women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113. [Google Scholar] [CrossRef]
- Lee, S.H.; Gong, H.S. Measurement and Interpretation of Handgrip Strength for Research on Sarcopenia and Osteoporosis. J. Bone Metab. 2020, 27, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zaccagni, L.; Toselli, S.; Bramanti, B.; Gualdi-Russo, E.; Mongillo, J.; Rinaldo, N. Handgrip Strength in Young Adults: Association with Anthropometric Variables and Laterality. Int. J. Environ. Res. Public Health 2020, 17, 4273. [Google Scholar] [CrossRef]
- Moradi, A.; Damirchi, E.S.; Narimani, M.; Esmaeilzadeh, S.; Dziembowska, I.; Azevedo, L.B.; Prado, W.L.D. Association between Physical and Motor Fitness with Cognition in Children. Medicina 2019, 55, 7. [Google Scholar] [CrossRef] [Green Version]
- Silverman, M.N.; Deuster, P.A. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus 2014, 4, 20140040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwyer, T.; Magnussen, C.G.; Schmidt, M.D.; Ukoumunne, O.C.; Ponsonby, A.-L.; Raitakari, O.T.; Zimmet, P.Z.; Blair, S.N.; Thomson, R.; Cleland, V.; et al. Decline in Physical Fitness from Childhood to Adulthood Associated with Increased Obesity and Insulin Resistance in Adults. Diabetes Care 2008, 32, 683–687. [Google Scholar] [CrossRef] [Green Version]
- García-Hermoso, A.; Alonso-Martinez, A.M.; Ramírez-Vélez, R.; Izquierdo, M. Effects of Exercise Intervention on Health-Related Physical Fitness and Blood Pressure in Preschool Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2020, 50, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Pazzianotto-Forti, E.M.; Moreno, M.A.; Plater, E.; Baruki, S.B.S.; Junior, I.R.; Reid, W.D. Impact of Physical Training Programs on Physical Fitness in People with Class II and III Obesity: A Systematic Review and Meta-Analysis. Phys. Ther. 2020, 100, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kodama, S.; Sugawara, A.; Saito, K.; Sone, H. Physical Fitness During Adolescence and Adult Mortality. Epidemiology 2009, 20, 463–464. [Google Scholar] [CrossRef]
- Sacheck, J. Pediatric Obesity: An Inflammatory Condition? J. Parenter. Enter. Nutr. 2008, 32, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Dumuid, D.; Olds, T.; Lewis, L.K.; Martin-Fernández, J.A.; Barreira, T.; Broyles, S.; Chaput, J.-P.; Fogelholm, M.; Hu, G.; Kuriyan, R.; et al. The adiposity of children is associated with their lifestyle behaviours: A cluster analysis of school-aged children from 12 nations. Pediatr. Obes. 2018, 13, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Buchan, D.S.; Thomas, N.E.; Baker, J.S. Novel risk factors of cardiovascular disease and their associations between obesity, physical activity and physical fitness. J. Public Health Res. 2012, 1, 11–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, K.; Jacobs, D.R.; Hong, C.-P.; Steinberger, J.; Moran, A.; Sinaiko, A. Association of physical activity with insulin sensitivity in children. Int. J. Obes. 2002, 26, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.-W.; Kwon, Y.-N.; Rhie, Y.-J.; Kim, H.-S.; Kim, Y.-S.; Paik, I.-Y.; Suh, S.-H.; Kim, D.-H. Effects of a Structured Exercise Program on Insulin Resistance, Inflammatory Markers and Physical Fitness in Obese Korean Children. J. Pediatr. Endocrinol. Metab. 2010, 23, 1065–1072. [Google Scholar] [CrossRef]
- Muntaner-Mas, A.; Palou, P.; Vidal-Conti, J.; Esteban-Cornejo, I. A Mediation Analysis on the Relationship of Physical Fitness Components, Obesity, and Academic Performance in Children. J. Pediatr. 2018, 198, 90–97. [Google Scholar] [CrossRef]
- Barry, V.W.; Baruth, M.; Beets, M.W.; Durstine, J.L.; Liu, J.; Blair, S.N. Fitness vs. Fatness on All-Cause Mortality: A Meta-Analysis. Prog. Cardiovasc. Dis. 2014, 56, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Muñoz, M.; Adsuar, J.; Pérez-Gómez, J.; Muñoz-Bermejo, L.; Garcia-Gordillo, M.; Carlos-Vivas, J. Influence of Body Composition on Physical Fitness in Adolescents. Medicina 2020, 56, 328. [Google Scholar] [CrossRef] [PubMed]
- Hussey, J.; Bell, C.; Bennett, K.; O’Dwyer, J.; Gormley, J. Relationship between the intensity of physical activity, inactivity, cardiorespiratory fitness and body composition in 7-10-year-old Dublin children. Br. J. Sports Med. 2007, 41, 311–316. [Google Scholar] [CrossRef]
- Chuang, H.-H.; Lin, R.-H.; Chen, J.-Y.; Yeh, W.-C.; Lin, H.-F.; Ueng, S.W.-N.; Hsu, K.-H. Effectiveness of a multi-faceted intervention among elementary school children. Medicina 2019, 98, e15079. [Google Scholar] [CrossRef] [PubMed]
- Sekgala, M.D.; Monyeki, K.D.; Mogale, M.A.; Ramoshaba, N.E. Performance of blood pressure to height ratio as a screening tool for elevated blood pressure in rural children: Ellisras Longitudinal Study. J. Hum. Hypertens. 2017, 31, 591–595. [Google Scholar] [CrossRef]
- Nafiu, O.O.; Burke, C.; Lee, J.; Voepel-Lewis, T.; Malviya, S.; Tremper, K.K. Neck Circumference as a Screening Measure for Identifying Children with High Body Mass Index. Pediatrics 2010, 126, e306–e310. [Google Scholar] [CrossRef]
- Chung, K.; Chiou, H.; Chang, J.; Chen, Y. Associations of nitric oxide with obesity and psychological traits among children and adolescents in Taiwan. Pediatr. Obes. 2019, 15, e12593. [Google Scholar] [CrossRef]
- Flegal, K.M.; Cole, T.J. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl. Health Stat. Rep. 2013, 2013, 1–3. [Google Scholar]
- Ma, W.-Y.; Yang, C.-Y.; Shih, S.-R.; Hsieh, H.-J.; Hung, C.S.; Chiu, F.-C.; Lin, M.-S.; Liu, P.-H.; Hua, C.-H.; Hsein, Y.-C.; et al. Measurement of Waist Circumference: Midabdominal or iliac crest? Diabetes Care 2013, 36, 1660–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1998. [Google Scholar]
- Grossi, M.; Riccò, B. Electrical impedance spectroscopy (EIS) for biological analysis and food characterization: A review. J. Sens. Sens. Syst. 2017, 6, 303–325. [Google Scholar] [CrossRef] [Green Version]
- Jensen, B.; Braun, W.; Geisler, C.; Both, M.; Klückmann, K.; Müller, M.J.; Bosy-Westphal, A. Limitations of Fat-Free Mass for the Assessment of Muscle Mass in Obesity. Obes. Facts 2019, 12, 307–315. [Google Scholar] [CrossRef]
- Hu, J.; Li, Z.; Li, S.; Li, H.; Wang, S.; Wang, S.; Han, L. Skipping breakfast and physical fitness among school-aged adolescents. Clinics 2020, 75, e1599. [Google Scholar] [CrossRef]
- Hsieh, P.-L.; Chen, M.-L.; Huang, C.-M.; Chen, W.-C.; Li, C.-H.; Chang, L.-C. Physical Activity, Body Mass Index, and Cardiorespiratory Fitness among School Children in Taiwan: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2014, 11, 7275–7285. [Google Scholar] [CrossRef] [Green Version]
- Hartman, J.G.; Looney, M. Norm-Referenced and Criterion-Referenced Reliability and Validity of the Back-Saver Sit-and-Reach. Meas. Phys. Educ. Exerc. Sci. 2003, 7, 71–87. [Google Scholar] [CrossRef]
- Lovecchio, N.; Novak, D.; Sedlacek, J.; Hamar, P.; Milanovic, I.; Radisavljevic-Janic, S.; Emeljanovas, A.; Eid, L.; Zago, M. Physical fitness for sedentary students: A common trend from six European countries. J. Sports Med. Phys. Fit. 2019, 59, 1389–1396. Available online: https://www.minervamedica.it/index2.php?show=R40Y2019N08A1389 (accessed on 4 May 2020). [CrossRef] [PubMed]
- Reid, R.E.; Fillon, A.; Thivel, D.; Henderson, M.; Barnett, T.A.; Bigras, J.-L.; Mathieu, M.-E. Can anthropometry and physical fitness testing explain physical activity levels in children and adolescents with obesity? J. Sci. Med. Sport 2020, 23, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Education. Measures for the Implementation of Physical Fitness Testing in Taiwan. 2018. Available online: https://edu.law.moe.gov.tw/LawContent.aspx?id=GL000769 (accessed on 28 March 2021).
- Templeton, G.F. A Two-Step Approach for Transforming Continuous Variables to Normal: Implications and Recommendations for IS Research. Commun. Assoc. Inf. Syst. 2011, 28, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lim, H. The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int. Rev. Psychiatry 2012, 24, 176–188. [Google Scholar] [CrossRef] [Green Version]
- NCD Risk Factor Collaboration (NCD-RisC). Heterogeneous contributions of change in population distribution of body mass index to change in obesity and underweight. Elife 2021, 10, e60060. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, A.; Zhou, B.; Sophiea, M.K.; Bentham, J.; Paciorek, C.J.; Iurilli, M.L.; Carrillo-Larco, R.M.; Bennett, J.E.; Di Cesare, M.; Taddei, C.; et al. Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: A pooled analysis of 2181 population-based studies with 65 million participants. Lancet 2020, 396, 1511–1524. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Stojanovic, M.D.; Stojanovic, V.; Maric, J.; Njaradi, N. Correlation between Fitness and Fatness in 6-14-year Old Serbian School Children. J. Health Popul. Nutr. 2011, 29, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Tomkinson, G.R.; Léger, L.A.; Olds, T.S.; Cazorla, G. Secular Trends in the Performance of Children and Adolescents (1980–2000). Sports Med. 2003, 33, 285–300. [Google Scholar] [CrossRef]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Olds, T.S.; Ridley, K.; Tomkinson, G.R. Declines in aerobic fitness: Are they only due to increasing fatness? Med. Sport Sci. 2007, 50, 226–240. [Google Scholar] [PubMed]
- Sacchetti, R.; Ceciliani, A.; Garulli, A.; Masotti, A.; Poletti, G.; Beltrami, P.; Leoni, E. Physical fitness of primary school children in relation to overweight prevalence and physical activity habits. J. Sports Sci. 2012, 30, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, G.R.; Olds, T.S. Secular Changes in Pediatric Aerobic Fitness Test Performance: The Global Picture. Med. Sport Sci. 2007, 50, 46–66. [Google Scholar]
- Zaqout, M.; Vyncke, K.; Moreno, L.A.; De Miguel-Etayo, P.; Lauria, F.; Molnar, D.; Lissner, L.; Hunsberger, M.; Veidebaum, T.; Tornaritis, M.; et al. Determinant factors of physical fitness in European children. Int. J. Public Health 2016, 61, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Lämmle, L.; Worth, A.; Bös, K. Socio-demographic correlates of physical activity and physical fitness in German children and adolescents. Eur. J. Public Health 2012, 22, 880–884. [Google Scholar] [CrossRef] [Green Version]
- Rosa-Guillamón, A.; Carrillo-López, P.J.; García-Cantó, E. Analysis of physical fitness according to sex, age, body mass index and level of physical activity in Spanish elementary school students. Rev. Fac. Med. 2020, 68, 92–99. [Google Scholar]
- Malina, R.M.; Bouchard, C. Growth, Maturation, and Physical Activity. Med. Sci. Sports Exerc. 1992, 24, 841. [Google Scholar] [CrossRef]
- Haywood, K.M.; Getchell, N. Life Span Motor Development; Human Kinetics: Champaign, IL, USA, 2019. [Google Scholar]
- Milanese, C.; Sandri, M.; Cavedon, V.; Zancanaro, C. The role of age, sex, anthropometry, and body composition as determinants of physical fitness in nonobese children aged 6-12. PeerJ 2020, 8, e8657. [Google Scholar] [CrossRef]
- Praagh, E.V. Anaerobic fitness tests: What are we measuring? Med. Sport Sci. 2007, 50, 26–45. [Google Scholar]
- Chu, N.-F. Prevalence and trends of obesity among school children in Taiwan—The Taipei Children Heart Study. Int. J. Obes. 2001, 25, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Chu, N.-F.; Pan, W.-H. Prevalence of obesity and its comorbidities among schoolchildren in Taiwan. Asia Pac. J. Clin. Nutr. 2007, 16, 601. [Google Scholar]
- Arfai, K.; Pitukcheewanont, P.D.; Goran, M.I.; Tavaré, C.J.; Heller, L.; Gilsanz, V. Bone, Muscle, and Fat: Sex-related Differences in Prepubertal Children. Radiology 2002, 224, 338–344. [Google Scholar] [CrossRef]
- Leonard, M.B.; Elmi, A.; Mostoufi-Moab, S.; Shults, J.; Burnham, J.M.; Thayu, M.; Kibe, L.; Wetzsteon, R.J.; Zemel, B.S. Effects of Sex, Race, and Puberty on Cortical Bone and the Functional Muscle Bone Unit in Children, Adolescents, and Young Adults. J. Clin. Endocrinol. Metab. 2010, 95, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Schierz, O.; Steinke, H.; Körner, A.; Kiess, W.; Kratzsch, J.; Hirsch, C. The LIFE Child study team; LIFE Child study team Sex hormones in association with general joint laxity and hypermobility in the temporomandibular joint in adolescents—results of the epidemiologic LIFE child study. J. Oral Rehabil. 2019, 46, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Garnett, S.P.; Högler, W.; Blades, B.; Baur, L.A.; Peat, J.; Lee, J.; Cowell, C.T. Relation between hormones and body composition, including bone, in prepubertal children. Am. J. Clin. Nutr. 2004, 80, 966–972. [Google Scholar] [CrossRef] [Green Version]
- Valdivia, O.D.; Ortega, F.Z.; Rodríguez, J.J.A.; Sánchez, M. Changes in flexibility according to gender and educational stage. Apunt. Med. l’esport 2009, 161, 10–17. [Google Scholar] [CrossRef]
- Golle, K.; Muehlbauer, T.; Wick, D.; Granacher, U. Physical Fitness Percentiles of German Children Aged 9–12 Years: Findings from a Longitudinal Study. PLoS ONE 2015, 10, e0142393. [Google Scholar] [CrossRef] [PubMed]
- Artero, E.G.; España-Romero, V.; Ortega, F.B.; Jimenez-Pavon, D.; Ruiz, J.R.; Vicente-Rodriguez, G.; Bueno, M.; Marcos, A.; Gómez-Martinez, S.; Urzanqui, A.; et al. Health-related fitness in adolescents: Underweight, and not only overweight, as an influencing factor. The AVENA study. Scand. J. Med. Sci. Sports 2010, 20, 418–427. [Google Scholar] [CrossRef]
- Xu, Y.; Mei, M.; Wang, H.; Yan, Q.; He, G. Association between Weight Status and Physical Fitness in Chinese Mainland Children and Adolescents: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 2468. [Google Scholar] [CrossRef] [Green Version]
- Casonatto, J.; Fernandes, R.A.; Batista, M.B.; Cyrino, E.S.; Coelho-E-Silva, M.J.; De Arruda, M.; Ronque, E.R.V. Association between health-related physical fitness and body mass index status in children. J. Child. Health Care 2016, 20, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.A.S.; Nunes, H.E.G. Prevalence and Factors Associated with Low Aerobic Performance Levels in Adolescents: A Systematic Review. Curr. Pediatr. Rev. 2015, 11, 56–70. [Google Scholar] [CrossRef]
- Zanini, D.; Kuipers, A.; Somensi, I.V.; Pasqualotto, J.F.; Quevedo, J.D.G.; Teo, J.C.; Antes, D.L. Relationship between body composition and physical capacities in junior soccer players. Rev. Bras. Cineantropometria Desempenho Humano 2020, 22, 22. [Google Scholar] [CrossRef]
- Ceschia, A.; Giacomini, S.; Santarossa, S.; Rugo, M.; Salvadego, D.; Da Ponte, A.; Driussi, C.; Mihaleje, M.; Poser, S.; Lazzer, S. Deleterious effects of obesity on physical fitness in pre-pubertal children. Eur. J. Sport Sci. 2016, 16, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Thivel, D.; Ring-Dimitriou, S.; Weghuber, D.; Frelut, M.-L.; O’Malley, G. Muscle Strength and Fitness in Pediatric Obesity: A Systematic Review from the European Childhood Obesity Group. Obes. Facts 2016, 9, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Winck, A.D.; Heinzmann-Filho, J.P.; Soares, R.B.; da Silva, J.S.; Woszezenki, C.T.; Zanatta, L.B. Effects of obesity on lung volume and capacity in children and adolescents: A systematic review. Rev. Paul Pediatr. 2016, 34, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Nzekwu, M.-M.U. The Effects of Body Mass Index on Lung Volumes. Chest 2006, 130, 827–833. [Google Scholar] [CrossRef]
- Davidson, W.J.; Mackenzie-Rife, K.A.; Witmans, M.B.; Montgomery, M.D.; Ball, G.D.; Egbogah, S.; Eves, N.D. Obesity negatively impacts lung function in children and adolescents. Pediatr. Pulmonol. 2014, 49, 1003–1010. [Google Scholar] [CrossRef]
- Dubern, B.; Tounian, P.; Medjadhi, N.; Maingot, L.; Girardet, J.-P.; Boulé, M. Pulmonary function and sleep-related breathing disorders in severely obese children. Clin. Nutr. 2006, 25, 803–809. [Google Scholar] [CrossRef]
- Brunet, M.; Chaput, J.-P.; Tremblay, A. The association between low physical fitness and high body mass index or waist circumference is increasing with age in children: The ‘Québec en Forme’ Project. Int. J. Obes. 2006, 31, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, P.M. Skeletal muscle physiology. Contin. Educ. Anaesth. Crit. Care Pain 2006, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ogborn, D.; Schoenfeld, B.J. The role of fiber types in muscle hypertrophy: Implications for loading strategies. Strength Cond. J. 2014, 36, 20–25. [Google Scholar] [CrossRef] [Green Version]
| Variables | Overall | Boys | Girls | p-Value a |
|---|---|---|---|---|
| Participants | n = 360 | n = 180 | n = 180 | |
| Demographic measures | ||||
| Age (years) | 10.0 ± 0.6 | 10.0 ± 0.7 | 10.0 ± 0.6 | 0.72 |
| Anthropometric measures | ||||
| Body mass index z-score | 0.366 ± 1.216 | 0.543 ± 1.269 | 0.190 ± 1.137 | 0.01 |
| Waist/height ratio | 0.46 ± 0.06 | 0.47 ± 0.06 | 0.45 ± 0.06 | <0.001 |
| Body composition measures | ||||
| Body-fat percentage | 16.9 ± 7.6 | 14.1 ± 8.0 | 19.5 ± 6.1 | <0.001 |
| Muscle weight (kg) | 29.0 ± 6.1 | 30.7 ± 5.6 | 27.5 ± 6.2 | <0.001 |
| Physical fitness levels | ||||
| 800-m run (sec) | 306.9 ± 69.4 | 297.1 ± 74.2 | 316.7 ± 63.0 | 0.01 |
| Sit-and-reach (cm) | 25.6 ± 8.4 | 23.1 ± 8.1 | 28.1 ± 7.9 | <0.001 |
| 1-min sit-ups (time) | 27.8 ± 8.8 | 28.7 ± 9.6 | 26.9 ± 7.9 | 0.049 |
| Standing long jump (cm) | 137.1 ± 25.6 | 141.1 ± 27.0 | 133.1 ± 23.5 | 0.003 |
| Predictors | Female Sex a | Age b | Body Mass Index z-Score b | Waist/Height Ratio b | Body-Fat Percentage b | Muscle Weight b | 800-m Run b | Sit-and-Reach b | 1-min Sit-Ups b | Standing Long Jump b |
|---|---|---|---|---|---|---|---|---|---|---|
| Female sex | – | |||||||||
| Age | 0.02 (0.72) | – | ||||||||
| Body mass index z-score | −0.14 (0.01 *) | −0.03 (0.63) | – | |||||||
| Waist/height ratio | −0.19 (<0.001 *) | −0.05 (0.37) | 0.80 (<0.001 *) | – | ||||||
| Body-fat percentage | 0.36 (<0.001 *) | 0.03 (0.57) | 0.69 (<0.001 *) | 0.62 (<0.001 *) | – | |||||
| Muscle weight | −0.24 (<0.001 *) | 0.44 (<0.001 *) | 0.68 (<0.001 *) | 0.43 (<0.001 *) | 0.43 (<0.001 *) | – | ||||
| 800-m run | 0.15 (0.004 *) | −0.04 (0.42) | 0.28 (<0.001 *) | 0.27 (<0.001 *) | 0.37 (<0.001 *) | 0.11 (0.04 *) | – | |||
| Sit-and-reach | 0.32 (<0.001 *) | 0.26 (<0.001 *) | −0.15 (0.004 *) | −0.15 (0.01 *) | 0.02 (0.69) | −0.07 (0.22) | −0.04 (0.51) | – | ||
| 1-min sit-ups | −0.10 (0.06) | 0.22 (<0.001 *) | −0.14 (0.01 *) | −0.18 (<0.001 *) | −0.28 (<0.001 *) | 0.01 (0.92) | −0.48 (<0.001 *) | 0.08 (0.11) | – | |
| Standing long jump | −0.15 (0.004 *) | 0.42 (<0.001 *) | −0.10 (0.06) | −0.11 (0.04 *) | −0.24 (<0.001 *) | 0.18 (0.001 *) | −0.39 (<0.001 *) | 0.27 (<0.001 *) | 0.44 (<0.001 *) | – |
| Predictors | B (95%CI) | p-Value a | B (95%CI) | p-Value a |
|---|---|---|---|---|
| Univariate Model | Multivariate Model | |||
| 800-m run | ||||
| Female sex | 19.6 (5.3–33.9) | 0.01 | NS | |
| Age | −4.5 (−15.5–6.5) | 0.42 | NI | |
| Body mass index z-score | 15.9 (10.2–21.6) | <0.001 | NS | |
| Waist/height ratio | 290.5 (180.7–400.2) | <0.001 | NS | |
| Body-fat percentage | 3.3 (2.4–4.2) | <0.001 | 3.4 (2.5–4.3) | <0.001 |
| Muscle weight | 1.3 (0.1–2.5) | 0.04 | NS | |
| Sit-and-reach | ||||
| Female sex | 5.0 (3.4–6.7) | <0.001 | 4.6 (3.0–6.3) | <0.001 |
| Age | 3.3 (2.0–4.6) | <0.001 | 3.1 (1.9–4.3) | <0.001 |
| Body mass index z-score | −1.1 (−1.8–−0.3) | 0.004 | −0.7 (−1.4–−0.1) | 0.03 |
| Waist/height ratio | −19.0 (−32.5–−5.5) | 0.01 | NS | |
| Body-fat percentage | 0.02 (−0.10–0.14) | 0.69 | NI | |
| Muscle weight | −0.1 (−0.2–0.1) | 0.22 | NI | |
| 1-min sit-ups | ||||
| Female sex | −1.8 (−3.7–−0.1) | 0.049 | NS | |
| Age | 2.9 (1.6–4.3) | <0.001 | 3.3 (2.0–4.7) | <0.001 |
| Body mass index z-score | −1.0 (−1.8–−0.3) | 0.01 | NS | |
| Waist/height ratio | −24.8 (−38.9–−10.7) | 0.001 | NS | |
| Body-fat percentage | −0.3 (−0.4–−0.2) | <0.001 | −0.3 (−0.4–−0.2) | <0.001 |
| Muscle weight | 0.1 (−0.1–0.2) | 0.92 | NI | |
| Standing long jump | ||||
| Female sex | −8.0 (−13.2–−2.7) | 0.003 | NS | |
| Age | 16.3 (12.6–20.0) | <0.001 | 14.1 (9.9–18.3) | <0.001 |
| Body mass index z-score | −2.1 (−4.3–0.1) | 0.06 | NS | |
| Waist/height ratio | −42.5 (−83.8–−1.1) | 0.04 | NS | |
| Body-fat percentage | −0.8 (−1.2–−0.5) | <0.001 | −1.1 (−1.4–−0.7) | <0.001 |
| Muscle weight | 0.8 (0.3–1.2) | 0.001 | 0.7 (0.2–1.2) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-Y.; Chen, L.-S.; Chang, I.-J.; Fang, W.-C.; Huang, S.-W.; Lin, R.-H.; Ueng, S.W.-N.; Chuang, H.-H. Can Anthropometry and Body Composition Explain Physical Fitness Levels in School-Aged Children? Children 2021, 8, 460. https://doi.org/10.3390/children8060460
Hsu C-Y, Chen L-S, Chang I-J, Fang W-C, Huang S-W, Lin R-H, Ueng SW-N, Chuang H-H. Can Anthropometry and Body Composition Explain Physical Fitness Levels in School-Aged Children? Children. 2021; 8(6):460. https://doi.org/10.3390/children8060460
Chicago/Turabian StyleHsu, Chih-Yu, Liang-Sien Chen, I-Jen Chang, Wei-Ching Fang, Sun-Weng Huang, Rong-Ho Lin, Steve Wen-Neng Ueng, and Hai-Hua Chuang. 2021. "Can Anthropometry and Body Composition Explain Physical Fitness Levels in School-Aged Children?" Children 8, no. 6: 460. https://doi.org/10.3390/children8060460
APA StyleHsu, C. -Y., Chen, L. -S., Chang, I. -J., Fang, W. -C., Huang, S. -W., Lin, R. -H., Ueng, S. W. -N., & Chuang, H. -H. (2021). Can Anthropometry and Body Composition Explain Physical Fitness Levels in School-Aged Children? Children, 8(6), 460. https://doi.org/10.3390/children8060460