Intrinsic Kidney Pathology Following COVID-19 Infection in Children and Adolescents: A Systematic Review
Abstract
:1. Introduction
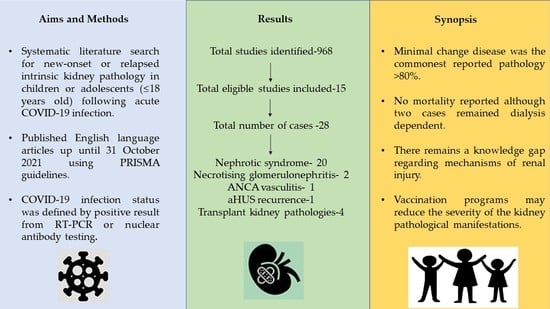
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Study Selection
2.3. Data Extraction
2.4. Study Registration
3. Results
3.1. New-Onset and Relapsed Nephrotic Syndrome
3.2. Glomerulonephritis and Hemolytic-Uremic Syndrome
3.3. Transplant Intrinsic Kidney Pathologies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Pneumonia of Unknown Cause. 2020. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229 (accessed on 10 November 2021).
- World Health Organization. WHO Coronavirus disease (COVID-19) dashboard. Available online: https://covid19.who.int/ (accessed on 10 November 2021).
- Jeyalan, V.; Storrar, J.; Wu, H.H.L.; Ponnusamy, A.; Sinha, S.; Kalra, P.A.; Chinnadurai, R. Native and transplant kidney histopathological manifestations in association with COVID-19 infection: A systematic review. World J. Transpl. 2021, 11, 480–502. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wu, J.; Hong, L.; Luo, Y.; Song, Q.; Chen, D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. Lancet Infect. Dis. 2020, 20, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Royal College of Paediatrics and Child Health: Guidance: Paediatric Multisystem Inflammatory Syndrome Temporally Associated with COVID-19. 2020. Available online: https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf (accessed on 10 November 2021).
- Godfred-Cato, S.; Bryant, B.; Leung, J.; Oster, M.E.; Conklin, L.; Abrams, J.; Roguski, K.; Wallace, B.; Prezzato, E.; Koumans, E.H.; et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- González-Dambrauskas, S.; Vásquez-Hoyos, P.; Camporesi, A.; Díaz-Rubio, F.; Piñeres-Olave, B.E.; Fernández-Sarmiento, J.; Gertz, S.; Harwayne-Gidansky, I.; Pietroboni, P.; Shein, S.L.; et al. Pediatric critical care and COVID-19. Pediatrics 2020, 146, e20201766. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol. 2020, 20, 453–454. [Google Scholar] [CrossRef]
- Alshami, A.; Roshan, A.; Catapang, M.; Jöbsis, J.J.; Kwok, T.; Polderman, N.; Sibley, J.; Sibley, M.; Mammen, C.; Matsell, D.G. Indications for kidney biopsy in idiopathic childhood nephrotic syndrome. Pediatr. Nephrol. 2017, 32, 1897–1905. [Google Scholar] [CrossRef]
- Alvarado, A.; Franceschi, G.; Resplandor, E.; Sumba, J.; Orta, N. COVID-19 associated with onset nephrotic syndrome in a pediatric patient: Coincidence or related conditions? Pediatr. Nephrol. 2021, 36, 205–207. [Google Scholar] [CrossRef]
- Shah, S.A.; Carter, H.P. New-onset nephrotic syndrome in a child associated with COVID-19 infection. Front. Pediatr. 2020, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Morreale, A.; Casciana, M.L. Onset of nephrotic syndrome concomitant to SARS-CoV-2 infection in a 3-year-old child. Pediatr. Nephrol. 2021, 37, 225. [Google Scholar] [CrossRef]
- Morgan, K.M.; Imani, P.D. Case report: A 5-year-old with new onset nephrotic syndrome in the setting of COVID-19 infection. BMC Nephrol. 2021, 22, 323. [Google Scholar] [CrossRef]
- Basalely, A.; Brathwaite, K.; Duong, M.D.; Liu, D.; Mazo, A.; Xie, Y.; Del Rio, M.; Goilav, B.; Hayde, N.; Kaskel, F.J.; et al. COVID-19 in children with kidney disease: A report of 2 cases. Kidney Med. 2021, 3, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Enya, T.; Morimoto, Y.; Oshima, R.; Miyazaki, K.; Miyazawa, T.; Okada, M.; Sugimoto, K. Nephrotic syndrome relapse in a boy with COVID-19. CEN Case Rep. 2021, 10, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Al Yazidi, L.; Al Nabhani, D. Coronavirus disease-2019 in children with nephrotic syndrome. Saudi J. Kidney Dis. Transpl. 2021, 32, 284–285. [Google Scholar] [CrossRef]
- Melgosa, M.; Madrid, A.; Alvárez, O.; Lumbreras, J.; Nieto, F.; Parada, E.; Perez-Beltrán, V. SARS-CoV-2 infection in Spanish children with chronic kidney pathologies. Pediatr. Nephrol. 2020, 35, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, S.; Mantan, M.; Mishra, K.; Kapoor, K.; Brijwal, M.; Kumar, M.; Sharma, S.; Swarnim, S.; Gaind, R.; Khandelwal, P.; et al. SARS-CoV-2 infection in children with chronic kidney disease. Pediatr. Nephrol. 2021; [In-Print]. [Google Scholar] [CrossRef]
- Basiratnia, M.; Derakhshan, D.; Yeganeh, B.S.; Derakhshan, A. Acute necrotizing glomerulonephritis associated with COVID-19 infection: Report of two pediatric cases. Pediatr. Nephrol. 2021, 36, 1019–1023. [Google Scholar] [CrossRef]
- Fireizen, Y.; Shahriary, C.; Imperial, M.E.; Randhawa, I.; Nianiaris, N.; Ovunc, B. Pediatric P-ANCA Vasculitis following COVID-19. Pediatr. Pulmonol. 2021, 56, 3422–3424. [Google Scholar] [CrossRef] [PubMed]
- Meshram, A.; Vala, K.B.; Saha, A.; Patel, H.V.; Kute, V.; Gera, D. Coronavirus Disease-2019 in Children with Primary Kidney Disease: A Case series. Saudi J. Kidney Dis. Transpl. 2021, 32, 218–222. [Google Scholar] [PubMed]
- Levenson, E.; Shepherd, T.N.; Aviles, D.; Craver, R.; Ehlayel, A.; Love, G.L.; Simms, K.J.; Straatmann, C.; Ashoor, I.F. De novo collapsing glomerulopathy in a pediatric kidney transplant recipient with COVID-19 infection. Pediatr. Transpl. 2021, 25, e14013. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.; Sekulic, M.; Kudose, S.; Kubin, C.; Ye, X.; Shayan, K.; Patel, A.; Cohen, D.J.; ERatner, L.; Santoriello, D.; et al. Kidney allograft biopsy findings after COVID-19. Am. J. Transpl. 2021; [In-Print]. [Google Scholar] [CrossRef] [PubMed]
- Berteloot, L.; Berthaud, R.; Temmam, S.; Lozach, C.; Zanelli, E.; Blanc, T.; Heloury, Y.; Capito, C.; Chardot, C.; Sarnacki, S.; et al. Arterial abnormalities identified in kidneys transplanted into children during the COVID-19 pandemic. Am. J. Transpl. 2021, 21, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Noone, D.G.; Iijima, K.; Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 2018, 392, 61–74. [Google Scholar] [CrossRef]
- Meyrier, A.; Niaudet, P. Acute kidney injury complicating nephrotic syndrome of minimal change disease. Kidney Int. 2018, 94, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A.; Symons, J.M. Nephrotic syndrome in childhood. Lancet 2003, 362, 629–639. [Google Scholar] [CrossRef]
- Ohtomo, Y.; Kawamura, R.; Kaneko, K.; Yamashiro, Y.; Kiyokawa, N.; Taguchi, T.; Mimori, K.; Fujimoto, J. Nephrotic syndrome associated with human parvovirus B19 infection. Pediatr. Nephrol. 2003, 18, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L.; Ferreira-Centeno, A.; Farkas-Szallasi, T.; Abraham, A.A.; Garrett, C.T. Viral DNA in microdissected renal biopsy tissue from HIV infected patients with nephrotic syndrome. Kidney Int. 1993, 43, 1347–1352. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Quigg, R.J. Glomerular diseases associated with hepatitis B and C. Adv. Chronic Kidney Dis. 2015, 22, 343–351. [Google Scholar] [CrossRef]
- Kari, J. Steroid-sensitive nephrotic syndrome and juvenile idiopathic arthritis. Pediatr. Nephrol. 2002, 11, 975–976. [Google Scholar] [CrossRef]
- Bilginer, Y.; Akpolat, T.; Ozen, S. Renal amyloidosis in children. Pediatr. Nephrol. 2011, 26, 1215–1227. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Tang, Y.; Xu, Y.; Yan, S.; Xu, Y.; Tan, L.; Zhong, Z.; Tarun, P.; Qin, W. The clinicopathological characteristics of Henoch-Schönlein purpura nephritis with presentation of nephrotic syndrome. Kidney Blood Press. Res. 2019, 44, 754–764. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.X.; Tang, F.; Zhu, H.Y.; Yi, F.; Yang, H.C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.P.; Bourne, T.D.; Wilson, J.D.; Saqqa, O.; Sharshir, M.D. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int. Rep. 2020, 5, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Brealey, J.K. Visualization of coronavirus in kidney. Kidney Int. 2020, 98, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Calomeni, E.; Satoskar, A.; Ayoub, I.; Brodsky, S.; Rovin, B.H.; Nadasdy, T. Multivesicular bodies mimicking SARS-CoV-2 in patients without COVID-19. Kidney Int. 2020, 98, 233–234. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Lombel, R.M.; Gipson, D.S.; Hodson, E.M. Treatment of steroid-sensitive nephrotic syndrome: New guidelines from KDIGO. Pediatr. Nephrol. 2013, 28, 415–426. [Google Scholar] [CrossRef]
- Albaqumi, M.; Barisoni, L. Current views on collapsing glomerulopathy. J. Am. Soc. Nephrol. 2008, 19, 1276–1281. [Google Scholar] [CrossRef] [Green Version]
- Albaqumi, M.; Soos, T.J.; Barisoni, L.; Nelson, P.J. Collapsing glomerulopathy. J. Am. Soc. Nephrol. 2006, 17, 2854–2863. [Google Scholar] [CrossRef]
- Detwiler, R.K.; Falk, R.J.; Hogan, S.L.; Jennette, J.C. Collapsing glomerulopathy: A clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int. 1994, 45, 1416–1424. [Google Scholar] [CrossRef] [Green Version]
- Laurinavicius, A.; Hurwitz, S.; Rennke, H.G. Collapsing glomerulopathy in HIV and non-HIV patients: A clinicopathological and follow-up study. Kidney Int. 1999, 56, 2203–2213. [Google Scholar] [CrossRef] [Green Version]
- Velez, J.C.; Caza, T.; Larsen, C.P. COVAN is the new HIVAN: The re-emergence of collapsing glomerulopathy with COVID-19. Nat. Rev. Nephrol. 2020, 16, 565–567. [Google Scholar] [CrossRef]
- Wu, H.; Larsen, C.P.; Hernandez-Arroyo, C.F.; Mohamed, M.M.B.; Caza, T.; Sharshir, M.; Chughtai, A.; Xie, L.; Gimenez, J.M.; Sandow, T.A.; et al. AKI and Collapsing Glomerulopathy Associated with COVID-19 and APOL 1 High-Risk Genotype. J. Am. Soc. Nephrol. 2020, 31, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Ossina, N.K.; Cannas, A.; Powers, V.C.; Fitzpatrick, P.A.; Knight, J.D.; Gilbert, J.R.; Shekhtman, E.M.; Tomei, L.D.; Umansky, S.R.; Kiefer, M.C. Interferon-gamma modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J. Biol. Chem. 1997, 272, 16351–16357. [Google Scholar] [CrossRef] [Green Version]
- Suso, A.S.; Mon, C.; Oñate Alonso, I.; Galindo Romo, K.; Juarez, R.C.; Ramírez, C.L.; Sánchez Sánchez, M.; Mercado Valdivia, V.; Ortiz Librero, M.; Oliet Pala, A.; et al. IgA Vasculitis with Nephritis (Henoch-Schönlein Purpura) in a COVID-19 Patient. Kidney Int. Rep. 2020, 5, 2074–2078. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020, 69, 1181–1189. [Google Scholar] [CrossRef]
- Uppal, N.N.; Kello, N.; Shah, H.H.; Khanin, Y.; De Oleo, I.R.; Epstein, E.; Sharma, P.; Larsen, C.P.; Bijol, V.; Jhaveri, K.D. De Novo ANCA-Associated Vasculitis with Glomerulonephritis in COVID-19. Kidney Int. Rep. 2020, 5, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.M.; Mastellos, D.C.; Huber-Lang, M.; Yancopoulou, D.; Garlanda, C.; Ciceri, F.; Lambris, J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020, 20, 343–344. [Google Scholar] [CrossRef] [Green Version]
- Gralinski, L.E.; Sheahan, T.P.; Morrison, T.E.; Menachery, V.D.; Jensen, K.; Leist, S.R.; Whitmore, A.; Heise, M.T.; Baric, R.S. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio 2018, 9, e01753-18. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhao, G.; Song, N.; Li, P.; Chen, Y.; Guo, Y.; Li, J.; Du, L.; Jiang, S.; Guo, R.; et al. Blockade of the C5a–C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg. Microbes Infect. 2018, 7, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, D.; Popoola, J.; Shah, S.; Ster, I.C.; Quan, V.; Phanish, M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020, 97, 1076–1082. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Marlais, M.; Wlodkowski, T.; Al-Akash, S.; Ananin, P.; Bandi, V.K.; Baudouin, V.; Boyer, O.; Vásquez, L.; Govindan, S.; Hooman, N.; et al. COVID-19 in children treated with immunosuppressive medication for kidney diseases. Arch. Dis. Child. 2021, 106, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Hartley, J.C.; Johnson, M.; Marks, S.D.; du Pré, P.; Stojanovic, J. Renal dysfunction in hospitalised children with COVID-19. Lancet Child Adolesc. Health 2020, 4, e28–e29. [Google Scholar] [CrossRef]
- Plouffe, B.; Van Hooren, T.; Barton, M.; Nashid, N.; Demirkaya, E.; Norozi, K.; Rachinsky, I.; Delport, J.; Knauer, M.; Tole, S.; et al. Renal Infarcts–A Perplexing Case in the Middle of the COVID-19 Pandemic. Front. Pediatr. 2021, 9, 669453. [Google Scholar] [CrossRef]
- Wu, H.H.L.; Kalra, P.A.; Chinnadurai, R. New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review. Vaccines 2021, 9, 125. [Google Scholar] [CrossRef] [PubMed]

| Author(s) and Country of Report | Age (yrs) | Sex | Ethnicity | Comorbidities | New-onset or Relapse | Clinical Presentation | Presentation Creatinine (mg/dL) | Presentation Proteinuria (g/day) | Presentation Albumin (g/dL) | Haematuria | Kidney Biopsy | Treatment Received | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alvarado et al. [10] Ecuador | 15 | M | Not Known | Nil | New-Onset | Anasarca, Dyspnoea, Oliguria | 0.55 | 3.9 | 1.5 | Nil | Not done as inpatient. To be scheduled as outpatient | Chloroquine and Azithromycin, daily boluses of methylprednisolone for 5 doses | Resolution of oedema |
| Shah et al. [11] United States | 8 | M | Not Known | Nil | New-Onset | Facial swelling, pedal/scrotal oedema | 0.32 | 11.4 | 2 | Yes, 2+ blood on urinalysis | No | Oral Prednisolone and supportive treatment | Achieved remission, continued oral prednisolone on reporting |
| Morreale et al. [12] Italy | 3 | Not Known | Italian, born to non-consanguineous parents | Nil | New-Onset | Abdominal distension/lower limb oedema | Not Known | 0.4 | 1.6 | Nil | No | Oral Prednisolone, Intravenous Albumin on Day 1, Furosemide from Day 3 | Prednisolone and furosemide were gradually tapered with disease remission |
| Morgan et al. [13] United States | 5 | F | Not Known | Nil | New-Onset | Abdominal distension/ lower limb oedema | 0.27 | >12 | 2 | Nil | No | Intravenous albumin and furosemide for diuresis, oral vitamin D and oral corticosteroids | Achieved complete remission within 3 weeks of starting corticosteroids and urine protein was still negative after 6 weeks of therapy |
| Basalely et al. [14] United States | Not Known | M | Hispanic | Steroid-sensitive Nephrotic Syndrome with infrequent relapses | Relapse | Anasarca | 0.5 | 18.7 | <2.0 | Moderate blood, 4–10 RBC, +hyaline casts | No | Received IV Abx. Blood Cultures +ve for Strep. Agalactiae, Stress-dose IV Hydrocortisone followed by oral Prednisolone, IV Albumin and IV Furosemide, prophylactic VTE treatment | Completed 10 days Abx treatment and 2 weeks of prophylactic VTE treatment alongside oral Prednisolone |
| Enya et al. [15] Japan | 3 | M | Japanese | Nephrotic Syndrome, Family Hx of Familial Hyper- cholesterolemia | Relapse | Eyelid oedema | 0.18 | 6.3 | 3.5 | Nil | No | Commenced on oral Prednisolone, otherwise supportive management | Achieved remission after a week of treatment |
| Al-Yazidi et al. [16] Oman | 10 | M | Arabic (Oman) | Steroid-sensitive Nephrotic Syndrome | Relapse | Facial edema, abdominal distension | Not Known | Not Known | Not Known | Nil | No | Commenced on oral Prednisolone, and required albumin infusion | Tapering of oral Prednisolone dose with resolution of proteinuria |
| Melgosa et al. [17] Spain (2 patients) | 2 patients with steroid-dependent nephrotic syndrome with acute COVID-19 infection provoked a relapse of their nephrotic syndrome. Both patients recovered following administration of oral Prednisolone without complications. Data were not described for each of these 2 patients individually. | ||||||||||||
| Krishnasamy et al. [18] India (11 patients) | 11 out of 24 patients with previous diagnosis of nephrotic syndrome developed relapse of their nephrotic syndrome following acute COVID-19 infection. Data and outcomes were not described for each of these 11 patients individually. | ||||||||||||
| Author and Country of Report | Age (yrs) | Sex | Ethnicity | Comorbidities | Pathology | New-onset or Relapse | Presentation Creatinine (mg/dL) | Presentation Proteinuria (g/Day) | Presentation Albumin (g/dL) | RBC per High Powered Field | Kidney Biopsy | Treatment Received | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basiratnia et al. [19] Iran | 17 | M | Not Known | Nil | Acute Necrotising Glomerulonephritis | New-Onset | 17 | 5.6 | 4 | 3+ blood in urinalysis, “many” RBCs | Yes | 3 doses of pulsed Methylprednisolone followed by Prednisolone and HD with VTE treatment | Discharged DD. |
| Basiratnia et al. [19] Iran | 16 | M | Not Known | Nil significant, but episode of gastroenteritis and fever 1 month prior to admission | Acute Necrotising Glomerulonephritis | New-Onset | 15.6 | Not described. Urinalysis 3+ protein | 4 | 2+ blood in urinalysis, “many” RBCs | Yes | 3 doses of pulsed Methylprednisolone followed by Prednisolone and 2 sessions of HD with VTE treatment | Resolution of AKI. Discharged DI. |
| Fireizen et al. [20] United States | 17 | M | Not Known | Obesity, Asthma, had COVID-19 pneumonia 2 months prior to presentation | pANCA (MPO) vasculitis | New-Onset | 0.78 1st admission, 1.30 2nd admission 1.52 3rd admission, all admissions shortly after one another | Not described | Not stated, but presence of visible proteinuria noted | Not stated, but presence of visible haematuria noted | Yes | Initial admission-Remdesivir, Dexamethasone, and Azithromycin Following vasculitis diagnosis (3rd admission): Methylprednisolone, Plasmapheresis, cyclophosphamide infusions and HD | Resolution Of AKI. Discharged DI. |
| Meshram et al. [21] India | 10 | M | Not Known | Nil | Recurrent antifactor H antibody-associated aHUS with underlying complement factor H-related protein mutation | Relapse | 2.9 | Not described. Urinalysis 3+ protein | Not described | Nil | No | Commenced on oral Prednisolone and MMF. Eventually received IV Rituximab (2 doses) and required IV immunoglobulin as well. Regular antihypertensive medications indicated. | Discharged DD. Patient progressed to CKD eventually and requires maintenance HD. Remained on regular oral Prednisolone |
| Author and Country of Report | Age (yrs) | Sex | Ethnicity | Comorbidities | Pathology | New-Onset or Relapse | Presentation Creatinine (mg/dL) | Presentation Proteinuria (g/Day) | Presentation Albumin (g/dL) | RBC per High Powered Field | Kidney Biopsy | Treatment Received | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Levenson et al. [22] United States | 16 | M | Black | Remote cerebrovascular accident, ESKD secondary to microscopic polyangitis (pANCA vasculitis), live-donor transplant recipient-previous acute antibody rejection | Collapsing Glomerulopathy | New-Onset | 2.3 and increasing to 4.7 (baseline 1.5) | 17 | 1.2 | Nil | Yes | Acute discontinuation of MMF, required two doses of IV immunoglobulin supportive treatment otherwise | Recovery of graft function, discharged DI, MMF increased back to regular doses |
| Daniel et al. [23] United States | 15 | F | Hispanic | ESKD secondary to decreased nephron mass. Patient received deceased donor kidney transplantation | T-cell-mediated rejection | New-Onset | 2.1 (baseline is 0.5) | 0.31 | 4 | 272 | Yes | Steroids and Bamlanvimab was administered as post COVID-19 therapy | Discharged with some recovery of graft function. |
| Berteloot et al. [24] France (2 patients) | 2 patients with positive COVID-19 RT-PCR results following kidney transplantation on day 2 and day 105, respectively, were described. Patient 1 had ESKD secondary to HUS, and received a deceased donor transplant. Patient 2 had CKDu, and also received a deceased donor transplant. Transplant kidney biopsy revealed <10% tubular interstitial infiltration in patient 1 and microcalcifications in patient 2. Both patients remained asymptomatic with the positive COVID-19 RT-PCR result. | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.H.L.; Shenoy, M.; Kalra, P.A.; Chinnadurai, R. Intrinsic Kidney Pathology Following COVID-19 Infection in Children and Adolescents: A Systematic Review. Children 2022, 9, 3. https://doi.org/10.3390/children9010003
Wu HHL, Shenoy M, Kalra PA, Chinnadurai R. Intrinsic Kidney Pathology Following COVID-19 Infection in Children and Adolescents: A Systematic Review. Children. 2022; 9(1):3. https://doi.org/10.3390/children9010003
Chicago/Turabian StyleWu, Henry H. L., Mohan Shenoy, Philip A. Kalra, and Rajkumar Chinnadurai. 2022. "Intrinsic Kidney Pathology Following COVID-19 Infection in Children and Adolescents: A Systematic Review" Children 9, no. 1: 3. https://doi.org/10.3390/children9010003
APA StyleWu, H. H. L., Shenoy, M., Kalra, P. A., & Chinnadurai, R. (2022). Intrinsic Kidney Pathology Following COVID-19 Infection in Children and Adolescents: A Systematic Review. Children, 9(1), 3. https://doi.org/10.3390/children9010003