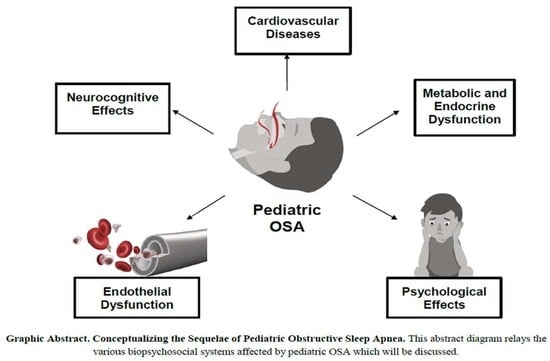
You Cannot Hit Snooze on OSA: Sequelae of Pediatric Obstructive Sleep Apnea
Abstract
:1. Introduction
2. Cardiovascular Diseases and OSA
2.1. Hypertension
2.2. Cardiac Arrhythmias
2.3. Coronary Artery Changes and Atherogenesis
2.4. Cerebrovascular Abnormalities
3. Endothelial Dysfunction
3.1. Inflammatory and Immune Markers
3.2. Renin–Angiotensin–Aldosterone System
3.3. Leptin
3.4. Hypercoagulability
3.5. Renal Injury and Microalbuminuria
3.6. Nonalcoholic Fatty Liver Disease
3.7. Gut Microbiota
4. Metabolic and Endocrine Dysfunction and OSA
4.1. Insulin Resistance
4.2. Inflammatory Markers
4.3. Metabolic Syndrome
4.4. Growth Failure
4.5. Polycystic Ovarian Syndrome
4.6. Testosterone Deficiency
4.7. Thyroid Function
5. Neurocognitive Abnormalities and OSA
5.1. Cortical Thinning
5.2. Problem Solving and Executive Function
5.3. Attention
5.4. Memory
5.5. School Performance
6. Psychological Syndromes and OSA
6.1. Anxiety
6.2. Depression
6.3. Behavior
6.4. Mood and Personality
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, E.; Kitamura, S.; Enomoto, M.; Moriwaki, A.; Kamio, Y.; Asada, T.; Arai, T.; Mishima, K. Prevalence of Childhood Obstructive Sleep Apnea Syndrome and Its Role in Daytime Sleepiness. PLoS ONE 2018, 13, e0204409. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, P.; Cocuzza, S.; Maniaci, A.; Ferlito, S.; Rasà, D.; Anzivino, R.; Vicini, C.; Iannella, G.; La Mantia, I. The Effect of Adenotonsillectomy on Children’s Behavior and Cognitive Performance with Obstructive Sleep Apnea Syndrome: State of the Art. Children 2021, 8, 921. [Google Scholar] [CrossRef] [PubMed]
- Gipson, K.; Lu, M.; Kinane, T.B. Sleep-Disordered Breathing in Children. Pediatrics Rev. 2019, 40, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Gozal, D. Pediatric OSA Syndrome Morbidity Biomarkers. Chest 2017, 151, 500–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangash, A.; Wajid, F.; Poolacherla, R.; Mim, F.K.; Rutkofsky, I.H. Obstructive Sleep Apnea and Hypertension: A Review of the Relationship and Pathogenic Association. Cureus 2020, 12, e8241. [Google Scholar] [CrossRef]
- Chan, K.C.-C.; Au, C.T.; Hui, L.L.; Wing, Y.K.; Li, A.M. Childhood OSA Is an Independent Determinant of Blood Pressure in Adulthood: Longitudinal Follow-up Study. Thorax 2020, 75, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Yacoub, M.; Youssef, I.; Salifu, M.O.; McFarlane, S.I. Cardiovascular Disease Risk in Obstructive Sleep Apnea: An Update. J. Sleep Disord. Ther. 2018, 7, 283. [Google Scholar] [CrossRef]
- Baker-Smith, C.M.; Isaiah, A.; Melendres, M.C.; Mahgerefteh, J.; Lasso-Pirot, A.; Mayo, S.; Gooding, H.; Zachariah, J. Sleep Disordered Breathing and Cardiovascular Disease in Children and Adolescents. J. Am. Heart Assoc. 2021, 10, e022427. [Google Scholar] [CrossRef]
- Burns, A.T.; Hansen, S.L.; Turner, Z.S.; Aden, J.K.; Black, A.B.; Hsu, D.P. Prevalence of Pulmonary Hypertension in Pediatric Patients with Obstructive Sleep Apnea and a Cardiology Evaluation: A Retrospective Analysis. J. Clin. Sleep Med. 2019, 15, 1081–1087. [Google Scholar] [CrossRef]
- Khositseth, A.; Chokechuleekorn, J.; Kuptanon, T.; Leejakpai, A. Rhythm disturbances in childhood obstructive sleep apnea during apnea-hypopnea episodes. Ann. Pediatr. Cardiol. 2013, 6, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Kraikriangsri, C.; Khositseth, A.; Kuptanon, T. P-Wave Dispersion as a Simple Tool for Screening Childhood Obstructive Sleep Apnea Syndrome. Sleep Med. 2019, 54, 159–163. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.M.; Serpero, L.D.; Tauman, R.; Gozal, D. Plasma Adhesion Molecules in Children with Sleep Disordered Breathing. Chest 2006, 129, 947–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halbower, A.C.; Degaonkar, M.; Barker, P.B.; Earley, C.J.; Marcus, C.L.; Smith, P.L.; Prahme, M.C.; Mahone, E.M. Childhood Obstructive Sleep Apnea Associates with Neuropsychological Deficits and Neuronal Brain Injury. PLoS Med. 2006, 3, e301. [Google Scholar] [CrossRef] [Green Version]
- Tabone, L.; Khirani, S.; Olmo Arroyo, J.; Amaddeo, A.; Griffon, L.; Sabil, A.; Fauroux, B. Cerebral Oxygenation during Respiratory Events in Children with Sleep-Disordered Breathing. Paediatr. Sleep Med. 2019, 214, 134–140. [Google Scholar] [CrossRef]
- Furtner, M.; Staudacher, M.; Frauscher, B.; Brandauer, E.; Esnaola y Rojas, M.M.; Gschliesser, V.; Poewe, W.; Schmidauer, C.; Ritsch-Marte, M.; Högl, B. Cerebral Vasoreactivity Decreases Overnight in Severe Obstructive Sleep Apnea Syndrome: A Study of Cerebral Hemodynamics. Sleep Med. 2009, 10, 875–881. [Google Scholar] [CrossRef]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep Apnea and Cardiovascular Disease. Circulation 2008, 118, 1080–1111. [Google Scholar] [CrossRef] [Green Version]
- Zhifei, X.; Fengjie, Z. Endothelial Dysfunction in Children with Obstructive Sleep Apnea Syndrome. Sleep Med. 2020, 58, 13–18. [Google Scholar] [CrossRef]
- Mochol, J.; Gawrys, J.; Gajecki, D.; Szahidewicz-Krupska, E.; Martynowicz, H.; Doroszko, A. Cardiovascular Disorders Triggered by Obstructive Sleep Apnea—A Focus on Endothelium and Blood Components. Int. J. Mol. Sci. 2021, 22, 5139. [Google Scholar] [CrossRef]
- Ceradini, D.J.; Kulkarni, A.R.; Callaghan, M.J.; Tepper, O.M.; Bastidas, N.; Kleinman, M.E.; Capla, J.M.; Galiano, R.D.; Levine, J.P.; Gurtner, G.C. Progenitor Cell Trafficking Is Regulated by Hypoxic Gradients through Hif-1 Induction of Sdf-1. Nat. Med. 2004, 10, 858–864. [Google Scholar] [CrossRef]
- Gozal, D.; Lipton, A.J.; Jones, K.L. Circulating Vascular Endothelial Growth Factor Levels in Patients with Obstructive Sleep Apnea. Sleep 2002, 25, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budhiraja, R.; Parthasarathy, S.; Quan, S.F. Endothelial Dysfunction in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2007, 03, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Møller, D. Abnormal Vasoactive Hormones and 24-Hour Blood Pressure in Obstructive Sleep Apnea. Am. J. Hypertens. 2003, 16, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Pace, A.; Iannella, G.; Rossetti, V.; Visconti, I.C.; Gulotta, G.; Cavaliere, C.; De Vito, A.; Maniaci, A.; Cocuzza, S.; Magliulo, G.; et al. Diagnosis of Obstructive Sleep Apnea in Patients with Allergic and Non-Allergic Rhinitis. Medicina 2020, 56, 454. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, G.; Iannella, G.; Vicini, C.; Polimeni, A.; Greco, A.; de Vincentiis, M.; Visconti, I.C.; Meccariello, G.; Cammaroto, G.; De Vito, A.; et al. Risk Factors for Obstructive Sleep Apnea Syndrome in Children: State of the Art. Int. J. Environ. Res. Public Health 2019, 16, 3235. [Google Scholar] [CrossRef] [Green Version]
- Zolotoff, C.; Bertoletti, L.; Gozal, D.; Mismetti, V.; Flandrin, P.; Roche, F.; Perek, N. Obstructive Sleep Apnea, Hyper-coagulability, and The Blood–Brain Barrier. J. Clin. Med. 2021, 10, 3099. [Google Scholar] [CrossRef]
- Li, X.; He, J. The Association between Serum/Plasma Leptin Levels and Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Endocrinol. 2021, 12, 1209. [Google Scholar] [CrossRef]
- Ding, C.; Lim, L.L.; Xu, L.; Kong, A.P. Sleep and Obesity. J. Obes. Metab. Syndr. 2018, 27, 4–24. [Google Scholar] [CrossRef] [Green Version]
- Varlami, V.; Malakasioti, G.; Alexopoulos, E.I.; Theologi, V.; Theophanous, E.; Liakos, N.; Daskalopoulou, E.; Gourgoulianis, K.; Kaditis, A.G. Low-grade albuminuria in children with obstructive sleep apnea. J. Sleep Res. 2013, 22, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Krishna, J.; Shah, Z.A.; Merchant, M.; Klein, J.B.; Gozal, D. Urinary Protein Expression Patterns in Children with Sleep Disordered Breathing: Preliminary Findings. Sleep Med. 2006, 7, 221–227. [Google Scholar] [CrossRef]
- Hwu, D.-W.; Lin, K.-D.; Lin, K.-C.; Lee, Y.-J.; Chang, Y.-H. The Association of Obstructive Sleep Apnea and Renal Outcomes —a Systematic Review and Meta-Analysis. BMC Nephrol. 2017, 18, 313. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.K.; Jang, M.J.; Kim, K.D.; Ahn, Y.M. The Association of Obstructive Sleep Apnea with Dyslipidemia in Korean Children and Adolescents: A Single Center, Cross-Sectional Study. J. Clin. Sleep Med. 2021, 17, 1599–1605. [Google Scholar] [CrossRef]
- Sundaram, S.S.; Halbower, A.; Pan, Z.; Robbins, K.; Capocelli, K.E.; Klawitter, J.; Shearn, C.T.; Sokol, R.J. Nocturnal Hypoxia-Induced Oxidative Stress Promotes Progression of Pediatric Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 65, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, S.S.; Halbower, A.C.; Klawitter, J.; Pan, Z.; Robbins, K.; Capocelli, K.E.; Sokol, R.J. Treating Obstructive Sleep Apnea and Chronic Intermittent Hypoxia Improves the Severity of Nonalcoholic Fatty Liver Disease in Children. J. Pediatrics 2018, 198, 67–75.e1. [Google Scholar] [CrossRef]
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am. J. Respir Crit. Care Med. 2019, 199, 830–841. [Google Scholar] [CrossRef]
- Umbro, I.; Fabiani, V.; Fabiani, M.; Angelico, F.; Ben, M.D. Association between Non-Alcoholic Fatty Liver Disease and Obstructive Sleep Apnea. World J. Gastroenterol. 2020, 26, 2669–2681. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Zheng, X.; Xia, Y.; Fu, Y.; Li, X.; Qian, Y.; Zou, J.; Zhao, A.; Guan, J.; et al. Pediatric Obstructive Sleep Apnea Is Associated with Changes in the Oral Microbiome and Urinary Metabolomics Profile: A Pilot Study. J. Clin. Sleep Med. 2018, 14, 1559–1567. [Google Scholar] [CrossRef]
- Azimian, F.; Ghiasi, F.; Amra, B.; Sebghatollahi, V. Association of Irritable Bowel Syndrome and Sleep Apnea in Patients Referred to Sleep Laboratory. J. Res. Med. Sci. 2017, 22, 72. [Google Scholar] [CrossRef]
- Green, B.T.; Broughton, W.A.; O’Connor, J.B. Marked Improvement in Nocturnal Gastroesophageal Reflux in a Large Cohort of Patients with Obstructive Sleep Apnea Treated with Continuous Positive Airway Pressure. Arch. Intern. Med. 2003, 163, 41. [Google Scholar] [CrossRef]
- Blechner, M.; Williamson, A.A. Consequences of Obstructive Sleep Apnea in Children. Curr. Probl. Pediatric Adolesc. Health Care 2016, 46, 19–26. [Google Scholar] [CrossRef]
- Punjabi, N.M.; Polotsky, V.Y. Disorders of Glucose Metabolism in Sleep Apnea. J. Appl. Physiol. 2005, 99, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Siriwat, R.; Wang, L.; Shah, V.; Mehra, R.; Ibrahim, S. Obstructive Sleep Apnea and Insulin Resistance in Children with Obesity. J. Clin. Sleep Med. 2020, 16, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Maddalozzo, J.; Sheldon, S.H.; Haymond, S.; Rychlik, K.; Lales, G.C.; Billings, K.R. Metabolic Alterations in Children with Obstructive Sleep Apnea. Int. J. Pediatric Otorhinolaryngol. 2014, 78, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Framnes, S.N.; Arble, D.M. The Bidirectional Relationship between Obstructive Sleep Apnea and Metabolic Dis-ease. Front. Endocrinol. 2018, 9, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, S.P.; Guleria, R.; Kabra, S.K. Metabolic Alterations and Systemic Inflammation in Overweight/Obese Children with Obstructive Sleep Apnea. PLoS ONE 2021, 16, e0252353. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Guilleminault, C.; Hwang, F.-M.; Cheng, C.; Lin, C.-H.; Li, H.-Y.; Lee, L.-A. Inflammatory Cytokines in Pediatric Obstructive Sleep Apnea. Medicine 2016, 95, e4944. [Google Scholar] [CrossRef]
- Bratel, T.; Wennlund, A.; Carlström, K. Pituitary Reactivity, Androgens and Catecholamines IN Obstructive Sleep Apnoea. Effects of Continuous Positive Airway Pressure Treatment (Cpap). Respir. Med. 1999, 93, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kaditis, A.G.; Alexopoulos, E.I.; Damani, E.; Karadonta, I.; Kostadima, E.; Tsolakidou, A.; Gourgoulianis, K.; Syrogi-annopoulos, G.A. Obstructive Sleep-Disordered Breathing and Fasting Insulin Levels in Nonobese Children. Pediatric Pulmonol. 2005, 40, 515–523. [Google Scholar] [CrossRef]
- Farr, O.M.; Rifas-Shiman, S.L.; Oken, E.; Taveras, E.M.; Mantzoros, C.S. Current Child, but Not Maternal, Snoring Is Bi-Directionally Related to Adiposity and Cardiometabolic Risk Markers: A Cross-Sectional and a Prospective Cohort Analysis. Metabolism 2017, 76, 70–80. [Google Scholar] [CrossRef]
- Gaines, J.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Bixler, E.O. Obstructive Sleep Apnea and the Metabolic Syndrome: The Road to Clinically-Meaningful Phenotyping, Improved Prognosis, and Personalized Treatment. Sleep Med. Rev. 2018, 42, 211–219. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Guilleminault, C.; Park, C.-S.; Son, H.-L.; Lee, H.-K.; Hwang, S.-H.; Choi, Y.-S. Changes in Salivary Cortisol Levels in Pediatric Patients with Obstructive Sleep Apnea Syndrome after Adenotonsillectomy. Sleep Med. 2014, 15, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Freezer, N.J.; Bucens, I.K.; Robertson, C.F. Obstructive Sleep Apnoea Presenting as Failure to Thrive in Infancy. Int. J. Pediatric Otorhinolaryngol. 1996, 34, 284. [Google Scholar] [CrossRef]
- Nieminen, P.; Lopponen, T.; Tolonen, U.; Lanning, P.; Knip, M.; Lopponen, H. Growth and Biochemical Markers of Growth in Children WITH Snoring and Obstructive Sleep Apnea. Pediatrics 2002, 109, e55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahal, H.; Tahrani, A.; Kyrou, I.; Dimitriadis, G.; Kimani, P.; Barber, T.; Nicholls, M.; Ali, A.; Weickert, M.; Randeva, H. The Relationship between Obstructive Sleep Apnoea and Quality of Life in Women with Polycystic Ovary Syndrome: A Cross-Sectional Study. Endocr. Abstr. 2018, 11, 2042018820906689. [Google Scholar] [CrossRef] [Green Version]
- Kahal, H.; Kyrou, I.; Tahrani, A.A.; Randeva, H.S. Obstructive Sleep Apnoea and Polycystic Ovary Syndrome: A Comprehensive Review of Clinical Interactions and Underlying Pathophysiology. Clin. Endocrinol. 2017, 87, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-D.; Cho, K.-S. Obstructive Sleep Apnea and Testosterone Deficiency. World J. Men’s Health 2019, 37, 12. [Google Scholar] [CrossRef]
- Briançon-Marjollet, A.; Weiszenstein, M.; Henri, M.; Thomas, A.; Godin-Ribuot, D.; Polak, J. The Impact of Sleep Disorders on Glucose Metabolism: Endocrine and Molecular Mechanisms. Diabetol. Metab. Syndr. 2015, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, K.; Baril, A.-A.; Gagnon, J.-F.; Fortin, M.; Décary, A.; Lafond, C.; Desautels, A.; Montplaisir, J.; Gosselin, N. Cognitive Impairment in Obstructive Sleep Apnea. Pathol. Biol. 2014, 62, 233–240. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, P.; Peng, Y.; Ouyang, R. Role of Oxidative Stress in the Neurocognitive Dysfunction of Obstructive Sleep Apnea Syndrome. Oxidative Med. Cell. Longev. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Alchanatis, M. Frontal Brain Lobe Impairment in Obstructive Sleep Apnoea: A Proton MR Spectroscopy Study. Eur. Respir. J. 2004, 24, 980–986. [Google Scholar] [CrossRef]
- Isaiah, A.; Ernst, T.; Cloak, C.C.; Clark, D.B.; Chang, L. Associations between Frontal Lobe Structure, Parent-Reported Obstructive Sleep Disordered Breathing and Childhood Behavior in the ABCD Dataset. Nat. Commun. 2021, 12, 2205. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.Y.; Jeon, S.; Kim, S.T.; Lee, J.-M.; Hong, S.B. Localized Cortical Thinning in Patients with Obstructive Sleep Apnea Syndrome. Sleep 2013, 36, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Ayalon, L.I.A.T.; Ancoli-Israel, S.O.N.I.A.; Drummond, S.E.A.N.P.A. Altered Brain Activation during Response Inhibition in Obstructive Sleep Apnea. J. Sleep Res. 2009, 18, 204–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, S.K.; Walczyk, J.J.; Buboltz, W.; Felix, V. The Relationship between Self-Reported Sleep Quality and Reading Comprehension Skills. Sleep Sci. 2014, 7, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Liu, G.; Luo, H.; Li, H.; Peng, Y.; Zong, D.; Ouyang, R. Aberrant Hippocampal Network Connectivity Is Associated with Neurocognitive Dysfunction in Patients with Moderate and Severe Obstructive Sleep Apnea. Front. Neurol. 2020, 11, 580408. [Google Scholar] [CrossRef] [PubMed]
- Krysta, K.; Bratek, A.; Zawada, K.; Stepańczak, R. Cognitive Deficits in Adults with Obstructive Sleep Apnea Compared to Children and Adolescents. J. Neural Transm. 2016, 124, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Karimi, M.; Hedner, J.; Zou, D.; Eskandari, D.; Lundquist, A.-C.; Grote, L. Attention Deficits Detected in Cognitive Tests Differentiate between Sleep Apnea Patients with or without a Motor Vehicle Accident. Sleep Med. 2015, 16, 528–533. [Google Scholar] [CrossRef]
- Verstraeten, E. Neurocognitive Effects of Obstructive Sleep Apnea Syndrome. Curr. Neurol. Neurosci. Rep. 2007, 7, 161–166. [Google Scholar] [CrossRef]
- Wallace, A.; Bucks, R.S. Memory and Obstructive Sleep Apnea: A Meta-Analysis. Sleep 2013, 36, 203–220. [Google Scholar] [CrossRef] [Green Version]
- Chinnadurai, S.; Jordan, A.K.; Sathe, N.A.; Fonnesbeck, C.; McPheeters, M.L.; Francis, D.O. Tonsillectomy for Obstructive Sleep-Disordered Breathing: A Meta-Analysis. Pediatrics 2017, 139, e20163491. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Chen, Y.-X.; Liu, L.; Yu, Z.-Y.; Luo, X. Neuropsychological Functioning after Adenotonsillectomy in Children with Obstructive Sleep Apnea: A Meta-Analysis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Waters, K.A.; Chawla, J.; Harris, M.-A.; Heussler, H.; Black, R.J.; Cheng, A.T.; Lushington, K. Cognition after Early Tonsillectomy for Mild Osa. Pediatrics 2020, 145, e20191450. [Google Scholar] [CrossRef] [PubMed]
- Olaithe, M.; Bucks, R.S. Executive Dysfunction in OSA before and after Treatment: A Meta-Analysis. Sleep 2013, 36, 1297–1305. [Google Scholar] [CrossRef]
- Pollicina, I.; Maniaci, A.; Lechien, J.R.; Iannella, G.; Vicini, C.; Cammaroto, G.; Cannavicci, A.; Magliulo, G.; Pace, A.; Cocuzza, S.; et al. Neurocognitive Performance Improvement after Obstructive Sleep Apnea Treatment: State of the Art. Behav. Sci. 2021, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Amdo, T.; Hasaneen, N.; Gold, M.S.; Gold, A.R. Somatic Syndromes, Insomnia, Anxiety, and Stress among Sleep Dis-ordered Breathing Patients. Sleep Breath. 2016, 20, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Macey, P.M.; Sarma, M.K.; Nagarajan, R.; Aysola, R.; Siegel, J.M.; Harper, R.M.; Thomas, M.A. Obstructive Sleep Apnea Is Associated with Low Gaba and High Glutamate in the Insular Cortex. J. Sleep Res. 2016, 25, 390–394. [Google Scholar] [CrossRef]
- Rezaeitalab, F.; Moharrari, F.; Saberi, S.; Asadpour, H.; Rezaeetalab, F. The Correlation of Anxiety and Depression with Obstructive Sleep Apnea Syndrome. J. Res. Med. Sci. 2014, 19, 205–210. [Google Scholar] [PubMed]
- Ejaz, S.M.; Khawaja, I.S.; Bhatia, S.; Hurwitz, T.D. Obstructive sleep apnea and depression: A review. Innov. Clin. Neurosci. 2011, 8, 17–25. [Google Scholar] [PubMed]
- Braitman, D.V. Screening for Sleep Apnea in Psychiatry. Am. J. Psychiatry Resid. J. 2018, 13, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, E.; Sedky, K.; Bennett, D.S. The relationship between depressive symptoms and OSA in pediatric population: A meta analysis. J. Clin. Sleep Med. 2013, 9, 1213–1220. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.; Edman, J.C.; Koltai, P.J. Obstructive sleep apnea in children. Am. Fam. Physician 2004, 69, 1147–1154. [Google Scholar] [CrossRef]
- Avior, G.; Fishman, G.; Leor, A.; Sivan, Y.; Kaysar, N.; DeRowe, A. The Effect of Tonsillectomy and Adenoidectomy on Inattention and Impulsivity as Measured by the Test of Variables of Attention (Tova) in Children with Obstructive Sleep Apnea Syndrome. Otolaryngol.–Head Neck Surg. 2004, 131, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. A Randomized Trial of Adenotonsillectomy for Childhood Sleep Apnea. N. Engl. J. Med. 2013, 368, 2366–2376. [Google Scholar] [CrossRef] [Green Version]
- Landau, Y.E.; Bar-Yishay, O.; Greenberg-Dotan, S.; Goldbart, A.D.; Tarasiuk, A.; Tal, A. Impaired Behavioral and Neurocognitive Function in Preschool Children with Obstructive Sleep Apnea. Pediatric Pulmonol. 2011, 47, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Isaiah, A.; Spanier, A.J.; Grattan, L.M.; Wang, Y.; Pereira, K.D. Predictors of Behavioral Changes after Adenotonsillectomy in Pediatric Obstructive Sleep Apnea. JAMA Otolaryngol.–Head Neck Surg. 2020, 146, 900. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Martinez, F.; Aguila, A.P.; Pal, A.; Aysola, R.S.; Henderson, L.A.; Macey, P.M. Stress in Obstructive Sleep Apnea. Sci. Rep. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Scarpina, F.; Bastoni, I.; Cappelli, S.; Priano, L.; Giacomotti, E.; Castelnuovo, G.; Molinari, E.; Tovaglieri, I.M.; Cornacchia, M.; Fanari, P.; et al. Psychological Well-Being in Obstructive Sleep Apnea Syndrome Associated with Obesity: The Relationship with Personality, Cognitive Functioning, and Subjective and Objective Sleep Quality. Front. Psychol. 2021, 12, 588767. [Google Scholar] [CrossRef]


| Affected System | Associated Sequelae |
|---|---|
| Cardiovascular | Hypertension, coronary artery disease, arrhythmias, cerebrovascular changes |
| Metabolic and endocrine | Insulin resistance, dyslipidemia, metabolic syndrome, growth changes, polycystic ovarian syndrome, testosterone deficiency, thyroid disorders |
| Endothelium | Vasoconstriction, atherosclerosis, hypercoagulability, renal damage |
| Gastrointestinal And Liver | liver disease, nonalcoholic fatty liver disease, irritable bowel syndrome, gastroesophageal reflux disease |
| Neurocognitive | Memory impairment, attention deficit hyperactivity disorder, executive function impairment |
| Psychosomatic | Anxiety, depression, behavioral issues |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.; Patel, S.; Gummalla, P.; Tablizo, M.A.; Kier, C. You Cannot Hit Snooze on OSA: Sequelae of Pediatric Obstructive Sleep Apnea. Children 2022, 9, 261. https://doi.org/10.3390/children9020261
Thomas S, Patel S, Gummalla P, Tablizo MA, Kier C. You Cannot Hit Snooze on OSA: Sequelae of Pediatric Obstructive Sleep Apnea. Children. 2022; 9(2):261. https://doi.org/10.3390/children9020261
Chicago/Turabian StyleThomas, Selena, Shefali Patel, Prabhavathi Gummalla, Mary Anne Tablizo, and Catherine Kier. 2022. "You Cannot Hit Snooze on OSA: Sequelae of Pediatric Obstructive Sleep Apnea" Children 9, no. 2: 261. https://doi.org/10.3390/children9020261
APA StyleThomas, S., Patel, S., Gummalla, P., Tablizo, M. A., & Kier, C. (2022). You Cannot Hit Snooze on OSA: Sequelae of Pediatric Obstructive Sleep Apnea. Children, 9(2), 261. https://doi.org/10.3390/children9020261