Wood Ash Based Treatment of Anaerobic Digestate: State-of-the-Art and Possibilities
Abstract
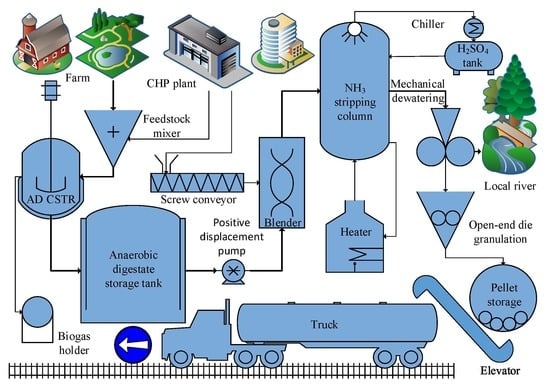
:1. Introduction
2. Upstream Processing
2.1. State-of-the-Art of AD
2.2. Use of WA as an Additive for Enhancing the AD
2.3. Stability and Maturity of the WA Amended Anaerobic Digestate

3. Downstream Processing
- Enhancement of AD could be achieved by preparing the feedstock with coal fly ash (CFA) using a dose as high as 2% (w/w) [76].
- Precipitation of struvite and adsorption of phosphate achieved by preparing a suspension with up to 3% (v/w) ash in swine wastewater [77].
- Supplementation of the anaerobic digestate by means of a WA dose up to 9.99% (w/w) or 3.09 g TS ash/g TS digestate to improve the nutrient ratio (C:N:P), the availability of phosphorus, and the microbial activity in the soil [60].
- Agreement with the regulations regarding the maximum content of heavy metals present in the anaerobic digestate [46,83]. The share of WA should not be greater than 15.51% (w/w) relative to the anaerobic digestate or 1.47 g TS WA/g TS AD. These results were obtained by considering the maximum content of heavy metals in the WA values established in the UK Quality Protocol of PL ash [84]. The content of Zn was found to be the limiting factor. The assumption of these calculations (Tables S1 and S2) are described in the Supplementary Material.
- Prevention of a large volume of dewatered digestate obtained via filtration by using as much CFA as the dry matter of the digestate (i.e., 1 g TS CFA/g TS digestate) to assist the dewatering process [28].
- Moure Abelenda et al. [85,86,87,88] tested alkaline and acid conditions to minimize the volatilization of NH3, and carbon and PO43− solubilization. They obtained better results (i.e., lower availability of nitrogen, carbon, and phosphorus) under acid conditions (4.39 g TS WA/g TS digestate) than under alkaline conditions (5.51 g TS WA/g TS digestate).
- Alkaline stabilization of sewage sludge via liming with a dose of CaO as high as 40% (w/w) or 8 g TS CaO/g TS sewage sludge to decrease the pathogens (Méndez et al., 2002). A dose of 3.82 g TS CaO/g TS digestate or 224.5 g CaO/L digestate (5.88% TS) was required for reaching a pH 12 and removing 51.2% of the NH4+-N due to NH3 volatilization [74]. Limoli et al. [74] reported that a low dose of 45 g/L increased the TS content of the manure digestate by 42.7%. When the organic material had higher dry matter (25.4% TS content), a dose of 50 g CaO/kg SS represented an increase in the TS content of approximately 30% and just 2 units of pH. This liming effect reduced the availability of heavy metals in the SS [89].
- Reducing phosphate availability by adding 5.6 kg of CFA to each kilogram of dairy slurry [90] could present a dose of greater than 110 g TS CFA/g TS slurry if the moisture content of the organic manure is 95%.
- Preparation of granules prepared with 100% (w/w) biomass ash showed the best mechanical properties. Decreasing the content to 80% bio ash and 20% dewatered SS (45% moisture) significantly affected the compressive strength of the pellets [29]. The lowest dose of bio ash and Ca(OH)2 that Pesonen et al. [29] tested corresponded to a 5.19 g TS bio ash + Ca(OH)2/g TS hygienized SS.
3.1. Pasteurization and Sterilization
3.2. Nitrogen Recovery Technologies
3.2.1. NH3 Stripping Processes from the WA Anaerobic Digestate
3.2.2. Manufacturing of (NH4)2CO3
3.2.3. Struvite Isolation Using WA as a Source of Magnesium
- Q (mol/L/g dry adsorbent), surface charge.
- W (g/L), dry mass of WBA-based adsorbent in the aqueous system (i.e., analyte).
- Ca (mol/L), concentration of the acid titrant in the aqueous system.
- [H+] & [OH−], concentration of H+ and OH− resulting from the direct measurement of the pH in the aqueous system (pH = −log([H+]); [H+]·[OH−] = 10−14).

3.3. Acidification of the Blend of WA and Anaerobic Digestate to Improve the Nutrient Management
3.3.1. Activation of the WA as Sorbent to Improve the Properties of the Blend with the Anaerobic Digestate as Slow-Released Fertilizer
3.3.2. Dewatering of the Blend of WA and Anaerobic Digestate
3.4. Commercial Processes for Manufacturing of a Granular Fertilizer based on Blends of Ash and Organic Manures
- Use of the most cost-efficient way of achieving solid-liquid separation.
- Find the optimum carbon and nutrient profile of the main stream coming out of the process, intended to be used as an organic amendment.
- Self-hardening to provide this material with the best mechanical properties before and after the granulation.
- Minimize any waste streams with valuable nutrients or other pollutants that need to be removed before disposal.
3.5. Inoculation of Biofertilizers in the Blended Fertilizer Prepared with Anaerobic Digestate and WA
- Vascular Arbuscular Mycorrhiza fungus:
- N-fixer bacterias can be symbiotic and non-symbiotic:
- Phosphate-solubilizing bacteria:
- Potassium-solubilizing bacteria:
- ○
- Bacillus mucilaginous [165].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NH4+-N | ammoniacal nitrogen |
| AD | anaerobic digestion |
| BET | Brunauer, Emmett, and Teller |
| BMP | biochemical methane potential |
| CUE | carbon use efficiency |
| C/N | carbon-to-nitrogen mas ratio |
| CTAB | cetyltrimethylammonium bromide |
| COD | chemical oxygen demand |
| EoW | end-of-waste |
| CFA | coal fly ash |
| EPS | extracellular polymeric substances |
| HRT | hydraulic retention time |
| MSW | municipal solid waste |
| OLR | organic loading rate |
| Corg | organic carbon |
| SS | sewage sludge |
| S/I | substrate to inoculum ration |
| TS | total solids |
| VFA | volatile fatty acids |
| VS | volatile solids |
| WS | water-soluble |
| WA | wood ash |
| WBA | wood bottom ash |
| WBA-H2O | wood bottom ash treated with de-ionized water |
| WBA-H2SO4 | wood bottom ash treated with sulfuric acid |
| WWTP | wastewater treatment plant |
References
- Banwart, S. Save our soils. Nature 2011, 474, 151–152. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.; Bennett, E. A broken biogeochemical cycle. Nature 2011, 478, 29–31. [Google Scholar] [CrossRef]
- Vaccari, D.A. Phosphorus: A Looming Crisis. Sci. Am. 2009, 300, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Hsu, E. Cost-benefit analysis for recycling of agricultural wastes in Taiwan. Waste Manag. 2021, 120, 424–432. [Google Scholar] [CrossRef]
- European Parliament. Directive 2018/851 Amending Directive 2008/98/EC on Waste Framework. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L.2018.150.01.0109.01.ENG (accessed on 14 December 2021).
- Corden, C.; Bougas, K.; Cunningham, E.; Tyrer, D.; Kreißig, J.; Zetti, E.; Gamero, E.; Wildey, R.; Crookes, M. Digestate and Compost as Fertilisers: Risk Assessment and Risk Management Options. Available online: https://ec.europa.eu/environment/chemicals/reach/pdf/40039%20Digestate%20and%20Compost%20RMOA%20-%20Final%20report%20i2_20190208.pdf (accessed on 14 December 2021).
- Saveyn, H.; Eder, P. End-of-Waste Criteria for Biodegradable Waste Subjected to Biological Treatment (Compost & Digestate): Technical Proposals. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC87124 (accessed on 14 December 2021).
- Victor, L. AD and Composting Industry Market Survey Report 2020. Available online: https://wrap.org.uk/sites/default/files/2021-01/AD%20%26%20Composting%20Market%20Survey%20Report.pdf (accessed on 14 December 2021).
- Wood, E.; James, K.; Barker, E. Comparison of the Environmental Impacts of Nitrogenous Materials. Available online: https://wrap.org.uk/sites/default/files/2021-01/Nitrogenous%20Materials%20Report%202020.pdf (accessed on 14 December 2021).
- Klages, S.; Heidecke, C.; Osterburg, B.; Bailey, J.; Calciu, I.; Casey, C.; Dalgaard, T.; Frick, H.; Glavan, M.; D’Haene, K.; et al. Nitrogen Surplus—A Unified Indicator for Water Pollution in Europe? Water 2020, 12, 1197. [Google Scholar] [CrossRef] [Green Version]
- Blumenthal, K. Generation and Treatment of Municipal Waste. Available online: https://op.europa.eu/en/publication-detail/-/publication/bd8a43dc-8076-4134-987d-c3081c8311e8 (accessed on 14 December 2021).
- Hou, Y.; Velthof, G.L.; Case, S.D.C.; Oelofse, M.; Grignani, C.; Balsari, P.; Zavattaro, L.; Gioelli, F.; Bernal, M.P.; Fangueiro, D.; et al. Stakeholder perceptions of manure treatment technologies in Denmark, Italy, the Netherlands and Spain. J. Clean. Prod. 2018, 172, 1620–1630. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Templeton, M.R. History and future of domestic biogas plants in the developing world. Energy Sustain. Dev. 2011, 15, 347–354. [Google Scholar] [CrossRef]
- Dagnall, S. UK strategy for centralised anaerobic digestion. Bioresour. Technol. 1995, 52, 275–280. [Google Scholar] [CrossRef]
- Madsen, M.; Holm-Nielsen, J.B.; Esbensen, K.H. Monitoring of anaerobic digestion processes: A review perspective. Renew. Sustain. Energy Rev. 2011, 15, 3141–3155. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part I. Phase-mineral transformations of organic and inorganic matter. Fuel 2013, 112, 391–449. [Google Scholar] [CrossRef]
- Pitman, R.M. Wood ash use in forestry—A review of the environmental impacts. Forestry 2006, 79, 563–588. [Google Scholar] [CrossRef] [Green Version]
- UK Forestry Commission. Forestry Statistics. Available online: https://www.forestresearch.gov.uk/tools-and-resources/statistics/forestry-statistics/forestry-statistics-2016-introduction/ (accessed on 14 December 2021).
- UK Quality Ash Association. UKQAA Ash Availability Report. Available online: http://www.ukqaa.org.uk/information/statistics/ (accessed on 14 December 2021).
- UK Quality Ash Association. About Us. Available online: http://www.ukqaa.org.uk/about/ (accessed on 14 December 2021).
- Andersson, L. Regular Recycling of Wood Ash to Prevent Waste Production. RecAsh. Available online: https://www.osti.gov/etdeweb/servlets/purl/20886561 (accessed on 14 December 2021).
- Guerrero, L.; Da Silva, C.; Barahona, A.; Montalvo, S.; Huiliñir, C.; Borja, R.; Peirano, C.; Toledo, M.; Carvajal, A. Fly ash as stimulant for anaerobic digestion: Effect over hydrolytic stage and methane generation rate. Water Sci. Technol. 2019, 80, 1384–1391. [Google Scholar] [CrossRef]
- Fernández-Delgado Juárez, M.; Waldhuber, S.; Knapp, A.; Partl, C.; Gómez-Brandón, M.; Insam, H. Wood ash effects on chemical and microbiological properties of digestate- and manure-amended soils. Biol. Fertil. Soils 2013, 49, 575–585. [Google Scholar] [CrossRef]
- Ibeto, C.N.; Lag-Brotons, A.J.; Marshall, R.; Semple, K.T. The Nutritional Effects of Digested and Undigested Organic Wastes Combined with Wood Ash Amendments on Carrot Plants. J. Soil Sci. Plant Nutr. 2020, 20, 460–472. [Google Scholar] [CrossRef]
- Fenton, O. Chemical Amendment of Slurry to Control Phosphorus Losses in Runoff. Available online: https://www.teagasc.ie/media/website/publications/2011/5669-Chemical-Amendment-of-Slurry.pdf (accessed on 14 December 2021).
- Zheng, Y.; Ke, L.; Xia, D.; Zheng, Y.; Wang, Y.; Li, H.; Li, Q. Enhancement of digestates dewaterability by CTAB combined with CFA pretreatment. Sep. Purif. Technol. 2016, 163, 282–289. [Google Scholar] [CrossRef]
- Pesonen, J.; Kuokkanen, V.; Kuokkanen, T.; Illikainen, M. Co-granulation of bio-ash with sewage sludge and lime for fertilizer use. J. Environ. Chem. Eng. 2016, 4, 4817–4821. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrve, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Alavi-Borazjani, S.A.; Capela, I.; Tarelho, L.A.C. Valorization of biomass ash in biogas technology: Opportunities and challenges. Energy Rep. 2020, 6, 472–476. [Google Scholar] [CrossRef]
- Alavi-Borazjani, S.A.; Tarelho, L.A.C.; Capela, I. A Brief Overview on the Utilization of Biomass Ash in Biogas Production and Purification. Waste Biomass Valorization 2021, 12, 6375–6388. [Google Scholar] [CrossRef]
- Adeyanju, A.A. Effect of seeding of wood-ash on biogas production using pig waste and cassava peels. J. Eng. Appl. Sci. 2008, 3, 242–245. [Google Scholar]
- Onwosi, C.O.; Okereke, G.U. Effect of water dilution and nutrient supplements (wood ash, urea and poultry droppings) on biogas production from brewers spent grain. In Current Research Topics in Applied Microbiology and Microbial Biotechnology; World Scientific: Seville, Spain, 2009; pp. 232–235. [Google Scholar] [CrossRef]
- Lo, H.M.; Liu, M.H.; Pai, T.Y.; Liu, W.F.; Lin, C.Y.; Wang, S.C.; Banks, C.J.; Hung, C.H.; Chiang, C.F.; Lin, K.C.; et al. Biostabilization assessment of MSW co-disposed with MSWI fly ash in anaerobic bioreactors. J. Hazard. Mater. 2009, 162, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.M.; Kurniawan, T.A.; Sillanpää, M.E.T.; Pai, T.Y.; Chiang, C.F.; Chao, K.P.; Liu, M.H.; Chuang, S.H.; Banks, C.J.; Wang, S.C.; et al. Modeling biogas production from organic fraction of MSW co-digested with MSWI ashes in anaerobic bioreactors. Bioresour. Technol. 2010, 101, 6329–6335. [Google Scholar] [CrossRef] [PubMed]
- Podmirseg, S.M.; Seewald, M.S.A.; Knapp, B.A.; Bouzid, O.; Biderre-Petit, C.; Peyret, P.; Insam, H. Wood ash amendment to biogas reactors as an alternative to landfilling? A preliminary study on changes in process chemistry and biology. Waste Manag. Res. 2013, 31, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.; Pelkonen, M.; Lagerkvist, A. Co-digestion of sewage sludge and wood fly ash. Environ. Technol. 2020, 9, 106564. [Google Scholar] [CrossRef] [PubMed]
- Cimon, C.; Kadota, P.; Eskicioglu, C. Effect of biochar and wood ash amendment on biochemical methane production of wastewater sludge from a temperature phase anaerobic digestion process. Bioresour. Technol. 2020, 297, 122440. [Google Scholar] [CrossRef]
- Bachmann, N. Design and engineering of biogas plants. In The Biogas Handbook: Science, Production, and Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 191–211. [Google Scholar] [CrossRef]
- Linke, B. Kinetic study of thermophilic anaerobic digestion of solid wastes from potato processing. Biomass Bioenergy 2006, 30, 892–896. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Bernal, M.P. Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric. Ecosyst. Environ. 2012, 160, 15–22. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Thermophilic co-digestion of pig manure and crude glycerol: Process performance and digestate stability. J. Biotechnol. 2013, 166, 97–104. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Strosser, E. Methods for determination of labile soil organic matter: An overview. J. Agrobiol. 2011, 27, 49–60. [Google Scholar] [CrossRef]
- WRAP. BSI PAS 110:2014 Specification for Whole Digestate, Separated Liquor and Separated Fibre Derived from the Anaerobic Digestion of Source-Segregated Biodegradable Materials. 2014. Available online: https://wrap.org.uk/resources/guide/bsi-pas-110-producing-quality-anaerobic-digestate (accessed on 5 January 2022).
- Banks, C.J.; Haeven, S.; Zhang, Y.; Sapp, M. Review of the Application of the Residual Biogas Potential Test. Available online: http://www.organics-recycling.org.uk/uploads/article2652/PAS110%20digestate%20stability%20review.pdf (accessed on 14 December 2021).
- Walker, M.; Banks, C.; Heaven, S.; Frederickson, J. Residual Biogas Potential Test for Digestates. Available online: https://www.wrap.org.uk/sites/files/wrap/Residual%20Biogas%20Potential.pdf (accessed on 28 February 2017).
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar] [CrossRef]
- Dijkstra, P.; Salpas, E.; Fairbanks, D.; Miller, E.B.; Hagerty, S.B.; Jan, K.; Groenigen, V.; Hungate, B.A.; Marks, J.C.; Koch, G.W.; et al. High carbon use efficiency in soil microbial communities is related to balanced growth, not storage compound synthesis. Soil Biol. Biochem. 2015, 89, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Geyer, K.M.; Kyker-Snowman, E.; Grandy, A.S.; Frey, S.D. Microbial carbon use efficiency: Accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 2016, 127, 173–188. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef]
- UK Government. Quality Protocol: Compost—End of Waste Criteria for the Production and Use of Quality Compost from Source-Segregated Biodegradable Waste. Available online: https://www.gov.uk/government/publications/quality-protocol-for-the-production-and-use-of-compost-from-waste (accessed on 14 December 2021).
- WRAP. PAS 100:2011 Specification for Composted Materials. Available online: http://www.organics-recycling.org.uk/page.php?article=3483#:~:text=PAS%20100%3A2018%20requires%20producers%20to%20set%20up%20a,also%20relates%20to%20the%20new%20%C3%82%C2%91compost%20quality%C3%82%C2%92%20clause (accessed on 15 December 2021).
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon—nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Rincón, B.; Borja, R.; González, J.M.; Portillo, M.C.; Sáiz-Jiménez, C. Influence of organic loading rate and hydraulic retention time on the performance, stability and microbial communities of one-stage anaerobic digestion of two-phase olive mill solid residue. Biochem. Eng. J. 2008, 40, 253–261. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Marshall, R.; Lag-Brotons, A.J.; Semple, K.T.; Stutter, M. Phosphorus solubility changes following additions of bioenergy wastes to an agricultural soil: Implications for crop availability and environmental mobility. Geoderma 2021, 401, 115150. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Laohaprapanon, S.; Marques, M.; Hogland, W. Removal of organic pollutants from wastewater using wood fly ash as a low-cost sorbent. Clean—Soil Air Water 2010, 38, 1055–1061. [Google Scholar] [CrossRef]
- Leechart, P.; Nakbanpote, W.; Thiravetyan, P. Application of ‘waste’ wood-shaving bottom ash for adsorption of azo reactive dye. J. Environ. Manag. 2009, 90, 912–920. [Google Scholar] [CrossRef]
- Forbes, M.S.; Raison, R.J.; Skjemstad, J.O. Formation, transformation and transport of black carbon (charcoal) in terrestrial and aquatic ecosystems. Sci. Total Environ. 2006, 370, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Menon, A.; Wang, J.Y.; Giannis, A. Optimization of micronutrient supplement for enhancing biogas production from food waste in two-phase thermophilic anaerobic digestion. Waste Manag. 2017, 59, 465–475. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- AECOM Inc.; Metcal & Eddy Inc. Fundamentals of biological treatment. In Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; Tchobanoglous, G., Stensel, H.D., Tsuchihashi, R., Burton, F., Eds.; Mc Graw Hill: New York, NY, USA, 2014; p. 576. [Google Scholar]
- Shanmugam, P.; Horan, N.J. Simple and rapid methods to evaluate methane potential and biomass yield for a range of mixed solid wastes. Bioresour. Technol. 2009, 100, 471–474. [Google Scholar] [CrossRef]
- Traversi, D.; Villa, S.; Lorenzi, E.; Degan, R.; Gilli, G. Application of a real-time qPCR method to measure the methanogen concentration during anaerobic digestion as an indicator of biogas production capacity. J. Environ. Manag. 2012, 111, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Seadon, J.K. Sustainable waste management systems. J. Clean. Prod. 2010, 18, 1639–1651. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Z.; Petracchini, F.; Lu, C.; Li, Y.; Zhang, Z.; Paolini, V.; Zhang, H. Preparation of Slow-Release Insecticides from Biogas Slurry: Effectiveness of Ion Exchange Resin in the Adsorption and Release of Ammonia Nitrogen. Processes 2021, 9, 1461. [Google Scholar] [CrossRef]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia removal from raw manure digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valorization 2017, 8, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Abbas, Y.; Yun, S.; Wang, K.; Ali Shah, F.; Xing, T.; Li, B. Static-magnetic-field coupled with fly-ash accelerant: A powerful strategy to significantly enhance the mesophilic anaerobic-co-digestion. Bioresour. Technol. 2021, 327, 124793. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, D.D.; Li, J.; Guo, G.; Tang, S. Phosphate recovery from swine wastewater using plant ash in chemical crystallization. J. Clean. Prod. 2017, 168, 338–345. [Google Scholar] [CrossRef]
- Bougnom, B.P.; Niederkofler, C.; Knapp, B.A.; Stimpfl, E.; Insam, H. Residues from renewable energy production: Their value for fertilizing pastures. Biomass Bioenergy 2012, 39, 290–295. [Google Scholar] [CrossRef]
- Miranda, C.; Soares, A.S.; Coelho, A.C.; Trindade, H.; Teixeira, C.A. Environmental implications of stored cattle slurry treatment with sulphuric acid and biochar: A life cycle assessment approach. Environ. Res. 2021, 194, 110640. [Google Scholar] [CrossRef]
- Brennan, R.B.; Healy, M.G.; Fenton, O.; Lanigan, G.J. The effect of chemical amendments used for phosphorus abatement on greenhouse gas and ammonia emissions from dairy cattle slurry: Synergies and pollution swapping. PLoS ONE 2015, 10, e0111965. [Google Scholar] [CrossRef] [Green Version]
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Alternatives to sulfuric acid for slurry acidification: Impact on slurry composition and ammonia emissions during storage. J. Clean. Prod. 2016, 131, 296–307. [Google Scholar] [CrossRef]
- Regueiro, I.; Coutinho, J.; Gioelli, F.; Balsari, P.; Dinuccio, E.; Fangueiro, D. Acidification of raw and co-digested pig slurries with alum before mechanical separation reduces gaseous emission during storage of solid and liquid fractions. Agric. Ecosyst. Environ. 2016, 227, 42–51. [Google Scholar] [CrossRef]
- UK Government. Quality Protocol of Anaerobic Digestate. End of Waste Criteria for the Production and Use of Quality Outputs from Anaerobic Digestion of Source-Segregated Biodegradable Waste. Available online: https://www.gov.uk/government/publications/quality-protocol-anaerobic-digestate (accessed on 14 December 2021).
- UK Government. Quality Protocol Poultry Litter Ash. End of Waste Criteria for the Production and Use of Treated Ash from the Incineration of Poultry Litter, Feathers and Straw. Available online: https://www.gov.uk/government/publications/quality-protocol-poultry-litter-ash (accessed on 14 December 2021).
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Effects of Wood Ash-Based Alkaline Treatment on Nitrogen, Carbon, and Phosphorus Availability in Food Waste and Agro-Industrial Waste Digestates. Waste Biomass Valorization 2020, 12, 3355–3370. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Kinetic study of the stabilization of an agro-industrial digestate by adding wood fly ash. Chem. Eng. J. Adv. 2021, 7, 100127. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Impact of sulphuric, hydrochloric, nitric, and lactic acids in the preparation of a blend of agro-industrial digestate and wood ash to produce a novel fertiliser. J. Environ. Chem. Eng. 2021, 9, 105021. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.; Aggidis, G.; Aiouache, F. Alkaline Wood Ash, Turbulence, and Traps with Excess of Sulfuric Acid Do Not Strip Completely the Ammonia off an Agro-waste Digestate. Edelweiss Chem. Sci. J. 2021, 4, 19–24. [Google Scholar] [CrossRef]
- Jamali, M.K.; Kazi, T.G.; Arain, M.B.; Afridi, H.I.; Memon, A.R.; Jalbani, N.; SHAH, A. Use of Sewage Sludge After Liming as Fertilizer for Maize Growth. Pedosphere 2008, 18, 203–213. [Google Scholar] [CrossRef]
- Brennan, R.B.; Fenton, O.; Rodgers, M.; Healy, M.G. Evaluation of chemical amendments to control phosphorus losses from dairy slurry. Soil Use Manag. 2011, 27, 238–246. [Google Scholar] [CrossRef] [Green Version]
- UK Government. Using Animal by-Products at Compost and Biogas Sites. Available online: https://www.gov.uk/guidance/using-animal-by-products-at-compost-and-biogas-sites (accessed on 14 December 2021).
- Kim, M.; Ahn, Y.H.; Speece, R.E. Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res. 2002, 36, 4369–4385. [Google Scholar] [CrossRef]
- AECOM Inc.; Metcalf & Eddy Inc. Advanced alkaline stabilisation technologies. In Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; Tchobanoglous, G., Stensel, H.D., Tsuchihashi, R., Burton, F., Eds.; Mc Graw Hill: New York, NY, USA, 2014; pp. 1501–1502. [Google Scholar]
- UK Government. Sewage Sludge in Agriculture: Code of Practice for England, Wales and Northern Ireland. Available online: https://www.gov.uk/government/publications/sewage-sludge-in-agriculture-code-of-practice/sewage-sludge-in-agriculture-code-of-practice-for-england-wales-and-northern-ireland (accessed on 14 December 2021).
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Demeyer, A.; Voundi Nkana, J.C.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Ribbing, C. Environmentally friendly use of non-coal ashes in Sweden. Waste Manag. 2007, 27, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Nag, R.; Auer, A.; Nolan, S.; Russell, L.; Markey, B.K.; Whyte, P.; O’Flaherty, V.; Bolton, D.; Fenton, O.; Richards, K.G.; et al. Evaluation of pathogen concentration in anaerobic digestate using a predictive modelling approach (ADRISK). Sci. Total Environ. 2021, 800, 149574. [Google Scholar] [CrossRef] [PubMed]
- UK Government. Handling of Manure and Slurry to Reduce Antibiotic Resistance. Available online: https://www.gov.uk/guidance/handling-of-manure-and-slurry-to-reduce-antibiotic-resistance (accessed on 14 December 2021).
- Al-Mallahi, J.; Sürmeli, R.Ö.; Çalli, B. Recovery of phosphorus from liquid digestate using waste magnesite dust. J. Clean. Prod. 2020, 272, 122616. [Google Scholar] [CrossRef]
- Sakthivel, S.R.; Tilley, E.; Udert, K.M. Wood ash as a magnesium source for phosphorus recovery from source-separated urine. Sci. Total Environ. 2012, 419, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Numviyimana, C.; Warchoł, J.; Ligas, B.; Chojnacka, K. Nutrients Recovery from Dairy Wastewater by Struvite Precipitation Combined with Ammonium Sorption on Clinoptilolite. Materials 2021, 14, 5822. [Google Scholar] [CrossRef]
- Laureni, M.; Palatsi, J.; Llovera, M.; Bonmatí, A. Influence of pig slurry characteristics on ammonia stripping efficiencies and quality of the recovered ammonium-sulfate solution. J. Chem. Technol. Biotechnol. 2013, 88, 1654–1662. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Vaddella, V.K.; Hristov, A.N.; Joo, H.S. Measuring Concentrations of Ammonia in Ambient Air or Exhaust Air Stream using Acid Traps. J. Environ. Qual. 2009, 38, 647–653. [Google Scholar] [CrossRef]
- Barrett, J. Periodicity of aqueous chemistry I: S- and p-block chemistry. In Inorganic Chemistry in Aqueous Solution; Barrett, J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2003; pp. 98–123. [Google Scholar]
- Zumdahl, S.S. Peroxide Ion. Available online: https://www.britannica.com/science/peroxide-ion (accessed on 5 January 2022).
- Drapanauskaite, D.; Handler, R.M.; Fox, N.; Baltrusaitis, J. Transformation of Liquid Digestate from the Solid-Separated Biogas Digestion Reactor Effluent into a Solid NH4HCO3 Fertilizer: Sustainable Process Engineering and Life Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 580–588. [Google Scholar] [CrossRef]
- Jantsch, T.G.; Mattiasson, B. A simple spectrophotometric method based on pH-indicators for monitoring partial and total alkalinity in anaerobic processes. Environ. Technol. 2003, 24, 1061–1067. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Owamah, H.I.; Dahunsi, S.O.; Oranusi, U.S.; Alfa, M.I. Fertilizer and sanitary quality of digestate biofertilizer from the co-digestion of food waste and human excreta. Waste Manag. 2014, 34, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Mu, Z.X.; He, C.S.; Jiang, J.K.; Zhang, J.; Yang, H.Y.; Mu, Y. A modified two-point titration method for the determination of volatile fatty acids in anaerobic systems. Chemosphere 2018, 204, 251–256. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Liu, L.; Pang, C.; Wu, S.; Dong, R. Optimization and evaluation of an air-recirculated stripping for ammonia removal from the anaerobic digestate of pig manure. Process Saf. Environ. Prot. 2015, 94, 350–357. [Google Scholar] [CrossRef]
- Drosg, B.; Fuchs, W.; Al Seadi, T.; Madsen, M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing. Available online: http://task37.ieabioenergy.com/technical-brochures.html#form (accessed on 14 December 2021).
- Miles, A.; Ellis, T.G. Struvite precipitation potential for nutrient recovery from anaerobically treated wastes. Water Sci. Technol. 2001, 43, 259–266. [Google Scholar] [CrossRef]
- Campos, J.L.; Crutchik, D.; Franchi, Ó.; Pavissich, J.P.; Belmonte, M.; Pedrouso, A.; Mosquera-Corral, A.; Val del Río, Á. Nitrogen and Phosphorus Recovery from Anaerobically Pretreated Agro-Food Wastes: A Review. Front. Sustain. Food Syst. 2019, 2, 91. [Google Scholar] [CrossRef] [Green Version]
- Escudero, A.; Blanco, F.; Lacalle, A.; Pinto, M. Struvite precipitation for ammonium removal from anaerobically treated effluents. J. Environ. Chem. Eng. 2015, 3, 413–419. [Google Scholar] [CrossRef]
- Prywer, J.; Sieroń, L.; Czylkowska, A. Struvite Grown in Gel, Its Crystal Structure at 90 K and Thermoanalytical Study. Crystals 2019, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, S.; Lunkwitz, K.; Keßler, B.; Spiegler, U.; Killmann, E.; Jaeger, W. Adsorption and stability of colloidal silica. Colloids Surf. A Physicochem. Eng. Asp. 2000, 163, 17–27. [Google Scholar] [CrossRef]
- Shah, I.; Adnan, R.; Ngah, W.S.W.; Mohamed, N. Iron impregnated activated carbon as an efficient adsorbent for the removal of methylene blue: Regeneration and kinetics studies. PLoS ONE 2015, 10, e0122603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagi, S.; Fukushi, K. Removal of phosphate from solution by adsorption and precipitation of calcium phosphate onto monohydrocalcite. J. Colloid Interface Sci. 2012, 384, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.; Brison, A.; Randall, D.G. Calcium removal from stabilized human urine by air and CO2 bubbling. Water Res. 2021, 202, 117467. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. Methods of accelerating and optimizing process efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef] [Green Version]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-liquid separation of animal slurry in theory and practice. Sustain. Agric. 2009, 2, 953–986. [Google Scholar] [CrossRef]
- Shi, Y.; Parker, D.B.; Cole, N.A.; Auvermann, B.W.; Mehlhorn, J.E. Surface amendments to minimize ammonia emissions from beef cattle feedlots. Trans. Am. Soc. Agric. Eng. 2001, 44, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Vandré, R.; Clemens, J. Studies on the relationship between slurry pH, volatilization processes and the influence of acidifying additives. Nutr. Cycl. Agroecosyst. 1996, 47, 157–165. [Google Scholar] [CrossRef]
- Fan, M.; Brown, R.C.; Wheelock, T.D.; Cooper, A.T.; Nomura, M.; Zhuang, Y. Production of a complex coagulant from fly ash. Chem. Eng. J. 2005, 106, 269–277. [Google Scholar] [CrossRef]
- Kavanagh, I.; Burchill, W.; Healy, M.G.; Fenton, O.; Krol, D.J.; Lanigan, G.J. Mitigation of ammonia and greenhouse gas emissions from stored cattle slurry using acidifiers and chemical amendments. J. Clean. Prod. 2019, 237, 117822. [Google Scholar] [CrossRef]
- Fomenko, A.I.; Sokolov, L.I. A study of sorption of phosphate ions from aqueous solutions by wood ash. Russ. J. Appl. Chem. 2015, 88, 652–656. [Google Scholar] [CrossRef]
- Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y. Soil Nitrogen Sorption Using Charcoal and Wood Ash. Agronomy 2021, 11, 1801. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Boran, S.; Tanasie, C. A green approach for treatment of wastewater with manganese using wood ash. J. Chem. Technol. Biotechnol. 2020, 95, 1781–1789. [Google Scholar] [CrossRef]
- Rosenfeld, P.E.; Henry, C.L. Activated Carbon and Wood Ash Sorption of Wastewater, Compost, and Biosolids Odorants. Water Environ. Res. 2001, 73, 388–393. [Google Scholar] [CrossRef]
- Kavanagh, I.; Fenton, O.; Healy, M.G.; Burchill, W.; Lanigan, G.J.; Krol, D.J. Mitigating ammonia and greenhouse gas emissions from stored cattle slurry using agricultural waste, commercially available products and a chemical acidifier. J. Clean. Prod. 2021, 294, 126251. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Mor, S.; Chhoden, K.; Ravindra, K. Application of agro-waste rice husk ash for the removal of phosphate from the wastewater. J. Clean. Prod. 2016, 129, 673–680. [Google Scholar] [CrossRef]
- Park, N.D.; Michael Rutherford, P.; Thring, R.W.; Helle, S.S. Wood pellet fly ash and bottom ash as an effective liming agent and nutrient source for rye grass (Lolium perenne L.) and oats (Avena sativa). Chemosphere 2012, 86, 427–432. [Google Scholar] [CrossRef]
- Janiszewska, D.; Olchowski, R.; Nowicka, A.; Zborowska, M.; Marszałkiewicz, K.; Shams, M.; Giannakoudakis, D.A.; Anastopoulos, I.; Barczak, M. Activated biochars derived from wood biomass liquefaction residues for effective removal of hazardous hexavalent chromium from aquatic environments. GCB Bioenergy 2021, 13, 1247–1259. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Li, W.; Xu, X.; Zhong, Q. Adsorption removal of ammonium and phosphate from water by fertilizer controlled release agent prepared from wheat straw. Chem. Eng. J. 2011, 171, 1209–1217. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Ronga, D.; Mantovi, P.; Pacchioli, M.T.; Pulvirenti, A.; Bigi, F.; Allesina, G.; Pedrazzi, S.; Tava, A.; Dal Prà, A. Combined effects of dewatering, composting and pelleting to valorize and delocalize livestock manure, improving agricultural sustainability. Agronomy 2020, 10, 661. [Google Scholar] [CrossRef]
- Dinuccio, E.; Balsari, P.; Berg, W. GHG emissions during the storage of rough pig slurry and the fractions obtained by mechanical separation. Aust. J. Exp. Agric. 2008, 48, 93–95. [Google Scholar] [CrossRef]
- Dinuccio, E.; Gioelli, F.; Balsari, P.; Dorno, N. Ammonia losses from the storage and application of raw and chemo-mechanically separated slurry. Agric. Ecosyst. Environ. 2012, 153, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Dinuccio, E.; Berg, W.; Balsari, P. Gaseous emissions from the storage of untreated slurries and the fractions obtained after mechanical separation. Atmos. Environ. 2008, 42, 2448–2459. [Google Scholar] [CrossRef] [Green Version]
- Dinuccio, E.; Biagini, D.; Rosato, R.; Balsari, P.; Lazzaroni, C. Organic matter and nitrogen balance in rabbit fattening and gaseous emissions during manure storage and simulated land application. Agric. Ecosyst. Environ. 2019, 269, 30–38. [Google Scholar] [CrossRef]
- Gioelli, F.; Dinuccio, E.; Balsari, P. Residual biogas potential from the storage tanks of non-separated digestate and digested liquid fraction. Bioresour. Technol. 2011, 102, 10248–10251. [Google Scholar] [CrossRef] [PubMed]
- Gioelli, F.; Dinuccio, E.; Cuk, D.; Rollè, L.; Balsari, P. Acidification with sulfur of the separated solid fraction of raw and co-digested pig slurry: Effect on greenhouse gas and ammonia emissions during storage. Anim. Prod. Sci. 2016, 56, 343–349. [Google Scholar] [CrossRef]
- Balsari, P.; Airoldi, G.; Dinuccio, E.; Gioelli, F. Ammonia emissions from farmyard manure heaps and slurry stores-Effect of environmental conditions and measuring methods. Biosyst. Eng. 2007, 97, 456–463. [Google Scholar] [CrossRef]
- Pampuro, N.; Bagagiolo, G.; Priarone, P.C.; Cavallo, E. Effects of pelletizing pressure and the addition of woody bulking agents on the physical and mechanical properties of pellets made from composted pig solid fraction. Powder Technol. 2017, 311, 112–119. [Google Scholar] [CrossRef]
- Weigand, H.; Bertau, M.; Hübner, W.; Bohndick, F.; Bruckert, A. RecoPhos: Full-scale fertilizer production from sewage sludge ash. Waste Manag. 2013, 33, 540–544. [Google Scholar] [CrossRef]
- Fivelman, Q. ADFerTech: Granular Fertiliser from Anaerobic Digestate Liquor. Available online: http://www.adfertech.com/ (accessed on 14 December 2021).
- Jewiarz, M.; Wróbel, M.; Fraczek, J.; Mudryk, K.; Dziedzic, K. Digestate, ash and Trichoderm based fertilizer-production line concept design. MATEC Web Conf. 2018, 168, 04004. [Google Scholar] [CrossRef] [Green Version]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef] [PubMed]
- Steenari, B.M.; Lindqvist, O. Stabilisation of biofuel ashes for recycling to forest soil. Biomass Bioenergy 1997, 13, 39–50. [Google Scholar] [CrossRef]
- Illikainen, M.; Tanskanen, P.; Kinnunen, P.; Körkkö, M.; Peltosaari, O.; Wigren, V.; Österbacka, J.; Talling, B.; Niinimäki, J. Reactivity and self-hardening of fly ash from the fluidized bed combustion of wood and peat. Fuel 2014, 135, 69–75. [Google Scholar] [CrossRef]
- Zafari, A.; Hosein Kianmehr, M. Effect of Temperature, Pressure and Moisture Content on Durability of Cattle Manure Pellet in Open-end Die Method. J. Agric. Sci. 2012, 4, 203–208. [Google Scholar] [CrossRef]
- Alemi, H.; Kianmehr, M.H.; Borghaee, A.M. Effect of Pellet Processing of Fertilizer on Slow-Release Nitrogen in Soil. Asian J. Plant Sci. 2010, 9, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.R.; Watabe, M.; Stewart, T.A.; Millar, B.C.; Moore, J.E. Pelleted organo-mineral fertilisers from composted pig slurry solids, animal wastes and spent mushroom compost for amenity grasslands. Waste Manag. 2007, 27, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Mudryk, K.; Frączek, J.; Wróbel, M.; Jewiarz, M.; Dziedzic, K. Agglomeration of Ash-Based Fertilizer Mixtures from Biomass Combustion and Digestate. In Renewable Energy Sources: Engineering, Technology, Innovation; Krzysztof, M., Sebastian, W., Eds.; Springer: Cham, Switzerland, 2018; pp. 823–834. [Google Scholar]
- US EPA. Alkaline Stabilization of Biosolids. Available online: https://www.epa.gov/biosolids/fact-sheet-alkaline-stabilization-biosolids (accessed on 14 December 2021).
- Rajendran, K.; Devaraj, P. Biomass and nutrient distribution and their return of Casuarina equisetifolia inoculated with biofertilizers in farm land. Biomass Bioenergy 2004, 26, 235–249. [Google Scholar] [CrossRef]
- Wu, S.C.; Cao, Z.H.; Li, Z.G.; Cheung, K.C.; Wong, M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma 2005, 125, 155–166. [Google Scholar] [CrossRef]
- Aseri, G.K.; Jain, N.; Panwar, J.; Rao, A.V.; Meghwal, P.R. Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of Pomegranate (Punica granatum L.) in Indian Thar Desert. Sci. Hortic. 2008, 117, 130–135. [Google Scholar] [CrossRef]
- Mahfouz, S.A.; Shamf-Eldin, M.A. Effect of mineral vs. biofertilizer on growth, yield, and essential oil content of fennel (Foeniculum vulgare Mill.). Int. Agrophysics 2007, 21, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Singh Brar, B.; Singh, J.; Singh, G.; Kaur, G. Effects of Long Term Application of Inorganic and Organic Fertilizers on Soil Organic Carbon and Physical Properties in Maize–Wheat Rotation. Agronomy 2015, 5, 220–238. [Google Scholar] [CrossRef]
- Johansen, A.; Carter, M.S.; Jensen, E.S.; Hauggard-Nielsen, H.; Ambus, P. Effects of digestate from anaerobically digested cattle slurry and plant materials on soil microbial community and emission of CO2 and N2O. Appl. Soil Ecol. 2013, 63, 36–44. [Google Scholar] [CrossRef]






| Reference |
|
|
|---|---|---|
| [33] |
|
|
| [34] |
|
|
| [35] Cited by the 3 articles below employing WA in AD |
|
|
| [37] |
|
|
| [38] |
|
|
| [39] |
|
|
| Minimum Temperature | Minimum Time | Maximum |
|---|---|---|
| 57 °C | 5 h | 50 mm |
| Activation Procedure | Carbon Content | pH | Reactivity |
|---|---|---|---|
| Carbonization | Increased, due to loss of volatile compounds containing H, O, S, and N | Slightly increased, due to the accumulation of ash with alkali and alkaline elements | Decreased. Mainly charred materials and recalcitrant compounds |
| Carbonation | Increase, in the form of inorganic carbon | Decrease, due to the neutralization of the alkaline element with carbonic acid | Decreased because carbonates are less soluble than oxides. |
| Calcination | Decrease, since all volatile compounds have already been lost in the carbonization | Increase, due to the release of CO2 | Increase, due to the formation of oxides |
| Acidification | Decrease, due to the dissociation of the CO3 -C and subsequent CO2 emissions | Decrease, due to the dissociation of the commercial acids | Slightly increase 1 |
| Wash | Slightly decrease, due to the removal of impurities | Slightly decreased due to the solubilization of alkalis | Slightly decrease, due to extraction of oxides |
| Milling and sieving | Slightly increase. Might enhance carbonation reactions during storage | Slightly increase. Might enhance the reaction for acidic and alkaline salts | Increase, due to greater availability of the elements |
| Process | Raw Materials | Blending Ratio | Advantages | Disadvantages |
|---|---|---|---|---|
| RecoPhos [153]. | Sewage sludge and H3PO4. | Incineration to produce SS ash. Reaction of the SS ash with the H3PO4 using phosphorus molar ratio 1:12 of sewage sludge ash: H3PO4 | Marketable granular product with similar properties to the triple superphosphate | The NH3 volatilized during the drying is not recovered. Emission of nitrogen oxides during incineration. |
| ADFerTech [154]. | Anaerobic digestate, dolomite (CaMg(CO3)2), organic binders and coating. | Dolomite was added to the liquid fraction (>91% moisture) in a dose ranging from 10 to 200 g/L. | Improve the aesthetic properties of the liquid fraction of anaerobic digestate. Decrease the cost of transportation and storage. | Additives of the liquid fraction of the anaerobic digestate are suitable for land application. |
| Limoli et al. [74]. | Anaerobic digestate, CaO, and H2SO4. | >95% moisture of anaerobic digestate. Dose of CaO to operate the stripping at pH 10. | Recovery of the NH3 volatilized. Enable the self-hardening and granulation of the NH3-depleted organic amendment. | Low fluency to be employed in a traditional stripping column (i.e., packed tower). High COD content of the filtrate. |
| Zhengh et al. [28]. | Anaerobic digestate, CFA, and CTAB. | >93% moisture of anaerobic digestate Mass of CTAB up to half of the TS of the digestate Mass of CFA up to the TS of the digestate | Reduce the energy consumption of the filtration of anaerobic digestate. Possible to enhance the mechanical separation with adsorption. | Presence of CTAB and heavy metals of the CFA in the filtrate. High COD content in the filtrate. |
| Pesonen et al. [29] | Sewage sludge, wood-peat ash, and Ca(OH)2. | Up to 40% of SS (45% moisture) and up to 30% Ca(OH)2. The dose of ash can go up to 100%. | No need to include Ca(OH)2 to have high compressive strength. Low presence of heavy metals | Dewatering and sanitation The NH3 released is not captured |
| Jewiarz et al. [155]. | Anaerobic digestate and woody biomass. | 18–20% moisture of the anaerobic digestate. Up to 75% WA. | Save in energy for drying the anaerobic digestate by thermal drying in fueling the drum drier with the biomass to produce the ash. | The NH3 released is not captured. Not possible to include the biofertilizer together with the ash due to the high pH. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abelenda, A.M.; Aiouache, F. Wood Ash Based Treatment of Anaerobic Digestate: State-of-the-Art and Possibilities. Processes 2022, 10, 147. https://doi.org/10.3390/pr10010147
Abelenda AM, Aiouache F. Wood Ash Based Treatment of Anaerobic Digestate: State-of-the-Art and Possibilities. Processes. 2022; 10(1):147. https://doi.org/10.3390/pr10010147
Chicago/Turabian StyleAbelenda, Alejandro Moure, and Farid Aiouache. 2022. "Wood Ash Based Treatment of Anaerobic Digestate: State-of-the-Art and Possibilities" Processes 10, no. 1: 147. https://doi.org/10.3390/pr10010147
APA StyleAbelenda, A. M., & Aiouache, F. (2022). Wood Ash Based Treatment of Anaerobic Digestate: State-of-the-Art and Possibilities. Processes, 10(1), 147. https://doi.org/10.3390/pr10010147