Magnetic Iron Nanoparticles: Synthesis, Surface Enhancements, and Biological Challenges
Abstract
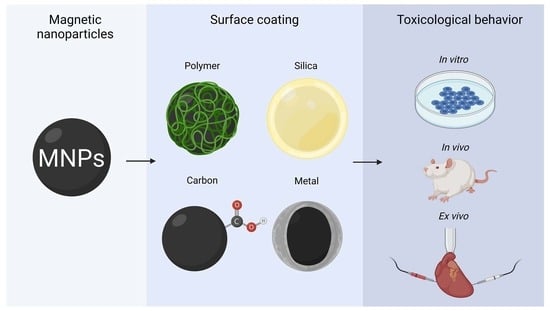
:1. Introduction
2. Magnetic Nanoparticle Synthesis Methods
2.1. Wet-Chemical Methods
2.1.1. Coprecipitation Synthesis
2.1.2. Thermal Decomposition
2.1.3. Hydrothermal Synthesis
2.1.4. Sol–Gel Synthesis
2.1.5. Microemulsion Synthesis
2.2. Assisted Methods
2.2.1. Sonochemically Assisted
2.2.2. Microwave-Assisted
2.3. Biological Synthesis Routes
2.4. Surface Coating
2.4.1. Silica Coating
2.4.2. Carbon-Based Coatings
2.4.3. Metallic Coatings
2.4.4. Polymer Coatings
2.5. Nanocomposites
3. Biological Challenges
3.1. In Vitro Toxicology
3.2. Ex Vivo Toxicity
3.3. In Vivo Toxicity
4. Regulation and Control
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Missaoui, W.N.; Arnold, R.D.; Cummings, B.S. Toxicological status of nanoparticles: What we know and what we don’t know. Chem. Biol. Interact. 2018, 295, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, F.; Mastropietro, T.F.; Poerio, T.; Godbert, N. Mesoporous TiO2 Thin Films: State of the Art. Titan. Dioxide-Mater. A Sustain. Environ. 2018, 508, 135–142. [Google Scholar]
- Madkour, L.H. Environmental Impact of Nanotechnology and Novel Applications of Nano Materials and Nano Devices; Springer: Cham, Germany, 2019; Volume 116. [Google Scholar]
- Prabha, S.; Arya, G.; Chandra, R.; Ahmed, B.; Nimesh, S. Effect of size on biological properties of nanoparticles employed in gene delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 83–91. [Google Scholar] [CrossRef]
- Gong, S.; Cheng, W. One-Dimensional Nanomaterials for Soft Electronics. Adv. Electron. Mater. 2017, 3, 1600314. [Google Scholar] [CrossRef]
- Navalón, S.; García, H. Nanoparticles for catalysis. Nanomaterials 2016, 6, 123. [Google Scholar] [CrossRef] [Green Version]
- Turci, F.; Pavan, C.; Leinardi, R.; Tomatis, M.; Pastero, L.; Garry, D.; Anguissola, S.; Lison, D.; Fubini, B. Revisiting the paradigm of silica pathogenicity with synthetic quartz crystals: The role of crystallinity and surface disorder. Part. Fibre Toxicol. 2016, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Selim, A.A.; Al-Sunaidi, A.; Tabet, N. Effect of the surface texture and crystallinity of ZnO nanoparticles on their toxicity. Mater. Sci. Eng. C 2012, 32, 2356–2360. [Google Scholar] [CrossRef]
- Usov, N.A.; Rytov, R.A.; Bautin, V.A. Properties of assembly of superparamagnetic nanoparticles in viscous liquid. Sci. Rep. 2021, 11, 1–11. [Google Scholar]
- Hu, M.; Butt, H.-J.; Landfester, K.; Bannwarth, M.B.; Wooh, S.; Thérien-Aubin, H. Shaping the Assembly of Superparamagnetic Nanoparticles. ACS Nano 2019, 13, 3015–3022. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Russek, S.E.; Zabow, G.; Sun, F.; Mohapatra, J.; Keenan, K.E.; Boss, M.A.; Zeng, H.; Liu, J.P.; Viert, A.; et al. Large T 1 contrast enhancement using superparamagnetic nanoparticles in ultra-low field MRI. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szpak, A.; Fiejdasz, S.; Prendota, W.; Strączek, T.; Kapusta, C.; Szmyd, J.; Nowakowska, M.; Zapotoczny, S. T1–T2 Dual-modal MRI contrast agents based on superparamagnetic iron oxide nanoparticles with surface attached gadolinium complexes. J. Nanoparticle Res. 2014, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shi, X.; Shen, M. Intelligent Design of Ultrasmall Iron Oxide Nanoparticle-Based Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 45119–45129. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.L.; Estrela-Lopis, I.; Böttner, J.; Lopes, C.A.P.; Guido, B.C.; de Souza, A.R.; Báo, S.N. Exploring cellular uptake of iron oxide nanoparticles associated with rhodium citrate in breast cancer cells. Int. J. Nanomed. 2017, 12, 5511–5523. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef]
- Kendall, M.; Ding, P.; Kendall, K. Particle and nanoparticle interactions with fibrinogen: The importance of aggregation in nanotoxicology. Nanotoxicology 2010, 5, 55–65. [Google Scholar] [CrossRef]
- Babakhani, P. The impact of nanoparticle aggregation on their size exclusion during transport in porous media: One- and three-dimensional modelling investigations. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 2014, 8, 233–278. [Google Scholar] [CrossRef]
- Sun, D.; Gong, L.; Xie, J.; Gu, X.; Li, Y.; Cao, Q.; Li, Q.; Luodan, A.; Gu, Z.; Xu, H. Toxicity of silicon dioxide nanoparticles with varying sizes on the cornea and protein corona as a strategy for therapy. Sci. Bull. 2018, 63, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Pope, C.A.; Cohen, A.J.; Burnett, R.T. Cardiovascular disease and fine particulate matter lessons and limitations of an integrated exposure-response approach. Circ. Res. 2018, 122, 1645–1647. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Particle Pollution and Cardiovascular Effects. 2021. Available online: https://www.epa.gov/pmcourse/particle-pollution-and-cardiovascular-effects (accessed on 24 February 2022).
- Schulz, H.; Harder, V.; Ibald-Mulli, A.; Khandoga, A.; Koenig, W.; Krombach, F.; Radykewicz, R.; Stampfl, A.; Thorand, B.; Peters, A. Cardiovascular Effects of Fine and Ultrafine Particles. J. Aerosol Med. 2005, 18, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Savliwala, S.; Chiu-Lam, A.; Unni, M.; Rivera-Rodriguez, A.; Fuller, E.; Sen, K.; Threadcraft, M.; Rinaldi, C. Magnetic Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- FDA. Information on Gadolinium-Based Contrast Agents Regulatory History and Labeling from Drugs @ FDA; FDA: Silver Spring, MD, USA, 2017. [Google Scholar]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Z.; Xie, J. Development of Manganese-Based Nanoparticles as Contrast Probes for Magnetic Resonance Imaging. Theranostics 2012, 2, 45–54. [Google Scholar] [CrossRef]
- De León-Rodríguez, L.M.; Martins, A.F.; Pinho, M.C.; Rofsky, N.M.; Sherry, A.D. Basic MR relaxation mechanisms and contrast agent design. J. Magn. Reson. Imaging 2015, 42, 545–565. [Google Scholar] [CrossRef] [Green Version]
- Neeley, C.; Moritz, M.; Brown, J.J.; Zhou, Y. Acute side effects of three commonly used gadolinium contrast agents in the paediatric population. Br. J. Radiol. 2016, 89, 20160027. [Google Scholar] [CrossRef] [Green Version]
- Watson, A.D. The use of gadolinium and dysprosium chelate complexes as contrast agents for magnetic resonance imaging This substituent group is believed to provide the required. J. Alloy. Compd. 1994, 207, 14–19. [Google Scholar] [CrossRef]
- Norek, M.; Peters, J.A. MRI contrast agents based on dysprosium or holmium. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 59, 64–82. [Google Scholar] [CrossRef]
- Urian, Y.; Atoche-Medrano, J.; Quispe, L.T.; Félix, L.L.; Coaquira, J. Study of the surface properties and particle-particle interactions in oleic acid-coated Fe3O4 nanoparticles. J. Magn. Magn. Mater. 2021, 525, 167686. [Google Scholar] [CrossRef]
- Kaur, I.P.; Kakkar, V.; Deol, P.K.; Yadav, M.; Singh, M.; Sharma, I. Issues and concerns in nanotech product development and its commercialization. J. Control Release 2014, 193, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liu, Z.; Li, L. Hybrid nanocomposites for imaging-guided synergistic theranostics. In Nanomaterials for Drug Delivery and Therapy; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 117–147. [Google Scholar]
- Oliveira, E.; Rocha, M.; Froner, A.P.; Basso, N.; Zanini, M.; Papaléo, R. Synthesis and nuclear magnetic relaxation properties of composite iron oxide nanoparticles. Quim. Nova 2018, 42, 57–64. [Google Scholar] [CrossRef]
- Williams, H.M. The application of magnetic nanoparticles in the treatment and monitoring of cancer and infectious diseases. Biosci. Horizons 2017, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lamon, L.; Asturiol, D.; Richarz, A.; Joossens, E.; Graepel, R.; Aschberger, K.; Worth, A. Grouping of nanomaterials to read-across hazard endpoints: From data collection to assessment of the grouping hypothesis by application of chemoinformatic techniques. Part. Fibre Toxicol. 2018, 15, 1–17. [Google Scholar] [CrossRef]
- Hensley, D.; Tay, Z.W.; Dhavalikar, R.; Zheng, B.; Goodwill, P.; Rinaldi, C.; Conolly, S. Combining magnetic particle imaging and magnetic fluid hyperthermia in a theranostic platform. Phys. Med. Biol. 2017, 62, 3483–3500. [Google Scholar] [CrossRef] [Green Version]
- Hapuarachchige, S.; Artemov, D. Theranostic Pretargeting Drug Delivery and Imaging Platforms in Cancer Precision Medicine. Front. Oncol. 2020, 10, 1131. [Google Scholar] [CrossRef]
- Thorat, N.D.; Lemine, O.M.; Bohara, R.A.; Omri, K.; El Mir, L.; Tofail, S.A.M. Superparamagnetic iron oxide nanocargoes for combined cancer thermotherapy and MRI applications. Phys. Chem. Chem. Phys. 2016, 18, 21331–21339. [Google Scholar] [CrossRef]
- Siddhardha, B.; Parasuraman, P. Theranostics application of nanomedicine in cancer detection and treatment. In Nanomaterials for Drug Delivery and Therapy; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 59–89. [Google Scholar]
- Kosuda, K.M.; Bingham, J.M.; Wustholz, K.L.; Van Duyne, R.P. Nanostructures and Surface-Enhanced Raman Spectroscopy. In Handbook of Nanoscale Optics and Electronicsvol; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; Volume 1–5. [Google Scholar]
- Morcos, B.; Lecante, P.; Morel, R.; Haumesser, P.H.; Santini, C.C. Magnetic, structural and chemical properties of cobalt nanoparticles synthesized in ionic liquids Bishoy. Langmuir 2018, 34, 7086–7095. [Google Scholar] [CrossRef]
- Ahghari, M.R.; Soltaninejad, V.; Maleki, A. Synthesis of nickel nanoparticles by a green and convenient method as a magnetic mirror with antibacterial activities. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]
- Patsula, V.; Tulinska, J.; Trachtová, Š.; Kuricova, M.; Liskova, A.; Španová, A.; Ciampor, F.; Vavra, I.; Rittich, B.; Ursinyova, M. Toxicity evaluation of monodisperse PEGylated magnetic nanoparticles for nanomedicine. Nanotoxicology 2019, 13, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Genevière, A.-M.; Derelle, E.; Escande, M.-L.; Grimsley, N.; Klopp, C.; Ménager, C.; Michel, A.; Moreau, H. Responses to iron oxide and zinc oxide nanoparticles in echinoderm embryos and microalgae: Uptake, growth, morphology, and transcriptomic analysis. Nanotoxicology 2020, 14, 1342–1361. [Google Scholar] [CrossRef]
- Guggenheim, E.J.; Rappoport, J.Z.; Lynch, I. Mechanisms for cellular uptake of nanosized clinical MRI contrast agents. Nanotoxicology 2020, 14, 504–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Brugger, J.; Pring, A. Mechanism and kinetics of hydrothermal replacement of magnetite by hematite. Geosci. Front. 2019, 10, 29–41. [Google Scholar] [CrossRef]
- Qiu, T.-S.; Fang, X.-H.; Wu, H.-Q.; Zeng, Q.-H.; Zhu, D.-M. Leaching behaviors of iron and aluminum elements of ion-absorbed-rare-earth ore with a new impurity depressant. Trans. Nonferrous Met. Soc. China 2014, 24, 2986–2990. [Google Scholar] [CrossRef]
- Strasser, H.; Brunner, H.; Schinner, F. Leaching of iron and toxic heavy metals from anaerobically-digested sewage sludge. J. Ind. Microbiol. Biotechnol. 1995, 14, 281–287. [Google Scholar] [CrossRef]
- Polasky, C.; Studt, T.; Steuer, A.-K.; Loyal, K.; Lüdtke-Buzug, K.; Bruchhage, K.-L.; Pries, R. Impact of Superparamagnetic Iron Oxide Nanoparticles on THP-1 Monocytes and Monocyte-Derived Macrophages. Front. Mol. Biosci. 2022, 9. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Li, J.; Du, C.; Huang, Z.; Chen, G.; Yan, W. Overendocytosis of superparamagnetic iron oxide particles increases apoptosis and triggers autophagic cell death in human osteosarcoma cell under a spinning magnetic field. Oncotarget 2016, 8, 9410–9424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Gan, M.; Yen, S.-H.; Dickson, D.W. Nanoparticles With Affinity for α-Synuclein Sequester α-Synuclein to Form Toxic Aggregates in Neurons With Endolysosomal Impairment. Front. Mol. Neurosci. 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Shukla, R.K.; Badiye, A.; Vajpayee, K.; Kapoor, N. Genotoxic Potential of Nanoparticles: Structural and Functional Modifications in DNA. Front. Genet. 2021, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.; Dunne, V.; Russell, B.; Mohamud, H.; Ghita, M.; McMahon, S.J.; Butterworth, K.T.; Schettino, G.; McGrry, C.K.; Prise, K.M. Impact of superparamagnetic iron oxide nanoparticles on in vitro and in vivo radiosensitisation of cancer cells. Radiat. Oncol. 2021, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cellai, F.; Munnia, A.; Viti, J.; Doumett, S.; Ravagli, C.; Ceni, E.; Mello, T.; Polvani, S.; Giese, R.W.; Baldi, G.; et al. Magnetic Hyperthermia and Oxidative Damage to DNA of Human Hepatocarcinoma Cells. Int. J. Mol. Sci. 2017, 18, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Döpke, C.; Grothe, T.; Steblinski, P.; Klöcker, M.; Sabantina, L.; Kosmalska, D.; Blachowicz, T.; Ehrmann, A. Magnetic Nanofiber Mats for Data Storage and Transfer. Nanomaterials 2019, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Grothe, T.; Sabantina, L.; Klöcker, M.; Junger, I.J.; Döpke, C.; Ehrmann, A. Wet Relaxation of Electrospun Nanofiber Mats. Technologies 2019, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Papavasileiou, A.; Panagiotopoulos, I.; Prodromidis, M.I. All-screen-printed graphite sensors integrating permanent bonded magnets. Fabrication, characterization and analytical utility. Electrochimica Acta 2020, 360, 136981. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, V.; Gupta, K.D. Performance analysis of flexible multirecess hydrostatic journal bearing operating with micropolar lubricant. Lubr. Sci. 2012, 24, 273–292. [Google Scholar] [CrossRef]
- Shahidi, S. Magnetic nanoparticles application in the textile industry—A review. J. Ind. Text. 2019, 50, 970–989. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Pardo, S.; Bucio, E. Interaction between Filler and Polymeric Matrix in Nanocomposites: Magnetic Approach and Applications. Polymers 2021, 13, 2998. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, M.; Miranda, J.A. Peak instability in an elastic interface ferrofluid. Phys. Fluids 2020, 32, 5. [Google Scholar] [CrossRef]
- Peyghami, A.; Moharrami, A.; Rashtbari, Y.; Afshin, S.; Vosuoghi, M.; Dargahi, A. Evaluation of the efficiency of magnetized clinoptilolite zeolite with Fe3O4 nanoparticles on the removal of basic violet 16 (BV16) dye from aqueous solutions. J. Dispers. Sci. Technol. 2021, 1–10. [Google Scholar] [CrossRef]
- Dargahi, A.; Hasani, K.; Mokhtari, S.A.; Vosoughi, M.; Moradi, M.; Vaziri, Y. Highly effective degradation of 2,4-Dichlorophenoxyacetic acid herbicide in a three-dimensional sono-electro-Fenton (3D/SEF) system using powder activated carbon (PAC)/Fe3O4 as magnetic particle electrode. J. Environ. Chem. Eng. 2021, 9, 105889. [Google Scholar] [CrossRef]
- Seidmohammadi, A.; Vaziri, Y.; Dargahi, A.; Nasab, H.Z. Improved degradation of metronidazole in a heterogeneous photo-Fenton oxidation system with PAC/Fe3O4 magnetic catalyst: Biodegradability, catalyst specifications, process optimization, and degradation pathway. Biomass Convers. Biorefinery 2021, 1–17. [Google Scholar] [CrossRef]
- Seabra, A.B.; Pelegrino, M.T.; Haddad, P.S. Antimicrobial Applications of Superparamagnetic Iron Oxide Nanoparticles: Perspectives and Challenges; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Blachowicz, T.; Ehrmann, A. Magnetization reversal in bent nanofibers of different cross sections. J. Appl. Phys. 2018, 124, 152112. [Google Scholar] [CrossRef] [Green Version]
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Elshemy, M.A. Iron Oxide Nanoparticles Versus Ferrous Sulfate In Treatment of Iron Deficiency Anemia In Rats. Egypt. J. Vet. Sci. 2018, 49, 103–109. [Google Scholar] [CrossRef]
- Wang, A.; Bagalkot, V.; Vasilliou, C.C.; Gu, F.; Alexis, F.; Zhang, L.; Shaikh, M.; Yuet, K.; Cima, M.J.; Langer, R.; et al. Superparamagnetic Iron Oxide Nanoparticle-Aptamer Bioconjugates for Combined Prostate Cancer Imaging and Therapy. ChemMedChem 2008, 3, 1311–1315. [Google Scholar] [CrossRef]
- Harrison, R.J.; Dunin-Borkowski, R.E.; Putnis, A. Direct imaging of nanoscale magnetic interactions in minerals. Proc. Natl. Acad. Sci. USA 2002, 99, 16556–16561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wáng, Y.X.J.; Idée, J.M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorat, N.D.; Bohara, R.A.; Malgras, V.; Tofail, S.A.M.; Ahamad, T.; Alshehri, S.M.; Wu, K.C.-W.; Yamauchi, Y. Multimodal Superparamagnetic Nanoparticles with Unusually Enhanced Specific Absorption Rate for Synergetic Cancer Therapeutics and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2016, 8, 14656–14664. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Shabanian, M.; Ghiyasi, S.; Vahabi, H.; Badawi, M.; Formela, K.; Puglia, D.; Saeb, M.R. Curing behavior of epoxy/Fe3O4nanocomposites: A comparison between the effects of bare Fe3O4, Fe3O4/SiO2/chitosan and Fe3O4/SiO2/chitosan/imide/phenylalanine-modified nanofillers. Prog. Org. Coat. 2018, 123, 10–19. [Google Scholar] [CrossRef]
- Darwish, M.; Kim, H.; Bui, M.; Le, T.-A.; Lee, H.; Ryu, C.; Lee, J.; Yoon, J. The Heating Efficiency and Imaging Performance of Magnesium Iron Oxide@tetramethyl Ammonium Hydroxide Nanoparticles for Biomedical Applications. Nanomaterials 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Stueber, D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef]
- Xu, L.; Zhong, S.; Shi, C.; Sun, Y.; Zhao, S.; Gao, Y.; Cui, X. Sonochemical fabrication of reduction-responsive magnetic starch-based microcapsules. Ultrason. Sonochem. 2018, 49, 169–174. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Campos, M.M. Metallic-nanoparticle release systems for biomedical implant surfaces: Effectiveness and safety. Nanotoxicology 2021, 15, 721–739. [Google Scholar] [CrossRef]
- Hu, T.; Mei, X.; Wang, Y.; Weng, X.; Liang, R.; Wei, M. Two-dimensional nanomaterials: Fascinating materials in biomedical field. Sci. Bull. 2019, 64, 1707–1727. [Google Scholar] [CrossRef] [Green Version]
- Gualdani, R.; Guerrini, A.; Fantechi, E.; Tadini-Buoninsegni, F.; Moncelli, M.R.; Sangregorio, C. Superparamagnetic iron oxide nanoparticles (SPIONs) modulate hERG ion channel activity. Nanotoxicology 2019, 13, 1197–1209. [Google Scholar] [CrossRef]
- Tian, F.; Chen, G.; Yi, P.; Zhang, J.; Li, A.; Zhang, J.; Zheng, L.; Deng, Z.; Shi, Q.; Peng, R.; et al. Fates of Fe3O4 and Fe3O4@SiO2 nanoparticles in human mesenchymal stem cells assessed by synchrotron radiation-based techniques. Biomaterials 2014, 35, 6412–6421. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Carmona, A.J.; Palomino-Ovando, M.A.; Hernández-Cristobal, O.; Sánchez-Mora, E.; Toledo-Solano, M. Synthesis and characterization of magnetic opal/Fe3O4 colloidal crystal. J. Cryst. Growth 2017, 462, 6–11. [Google Scholar] [CrossRef]
- Awada, H.; Al Samad, A.; Laurencin, D.; Gilbert, R.; Dumail, X.; El Jundi, A.; Bethry, A.; Pomrenke, R.; Johnson, C.; Lemaire, L.; et al. Controlled Anchoring of Iron Oxide Nanoparticles on Polymeric Nanofibers: Easy Access to Core@Shell Organic–Inorganic Nanocomposites for Magneto-Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 9519–9529. [Google Scholar] [CrossRef]
- Yazid, N.A.; Joon, Y.C. Co-precipitation synthesis of magnetic nanoparticles for efficient removal of heavy metal from synthetic wastewater Co-precipitation Synthesis of Magnetic Nanoparticles for Efficient Removal of Heavy Metal from Synthetic Wastewater. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2124, p. 020019. [Google Scholar]
- Daoush, W.M. Co-Precipitation and Magnetic Properties of Magnetite Nanoparticles for Potential Biomedical Applications. J. Nanomed. Res. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Mohammadi, H.; Nekobahr, E.; Akhtari, J.; Saeedi, M.; Akbari, J.; Fathi, F. Synthesis and characterization of magnetite nanoparticles by co-precipitation method coated with biocompatible compounds and evaluation of in-vitro cytotoxicity. Toxicol. Rep. 2021, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Cotin, G.; Kiefer, C.; Perton, F.; Ihiawakrim, D.; Blanco-Andujar, C.; Moldovan, S.; Lefevre, C.; Ersen, O.; Pichon, B.; Mertz, D.; et al. Unravelling the Thermal Decomposition Parameters for The Synthesis of Anisotropic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 881. [Google Scholar] [CrossRef] [Green Version]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Lassenberger, A.; Grünewald, T.A.; van Oostrum, P.D.J.; Rennhofer, H.; Amenitsch, H.; Zirbs, R.; Lichtenegger, H.C.; Reimhult, E. Monodisperse Iron Oxide Nanoparticles by Thermal Decomposition: Elucidating Particle Formation by Second-Resolved in Situ Small-Angle X-ray Scattering. Chem. Mater. 2017, 29, 4511–4522. [Google Scholar] [CrossRef] [Green Version]
- Torres-Gómez, N.; Nava, O.; Argueta-Figueroa, L.; García-Contreras, R.; Baeza-Barrera, A.; Vilchis-Nestor, A.R. Shape tuning of magnetite nanoparticles obtained by hydrothermal synthesis: Effect of temperature. J. Nanomater. 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ansar, M.Z.; Atiq, S.; Riaz, S.; Naseem, S. Magnetite Nano-crystallites for Anti-cancer Drug Delivery. Mater. Today Proc. 2015, 2, 5410–5414. [Google Scholar] [CrossRef]
- Sharafi, Z.; Bakhshi, B.; Javidi, J.; Adrangi, S. Synthesis of Silica-coated Iron Oxide Nanoparticles: Preventing Aggregation without Using Additives or Seed Pretreatment. Iran. J. Pharm. Res. IJPR 2018, 17, 386–395. [Google Scholar] [PubMed]
- Omelyanchik, A.; Salvador, M.; D’orazio, F.; Mameli, V.; Cannas, C.; Fiorani, D.; Musinu, A.; Rivas, M.; Rodionova, V.; Varvaro, G.; et al. Magnetocrystalline and surface anisotropy in cofe2o4 nanoparticles. Nanomaterials 2020, 10, 1288. [Google Scholar] [CrossRef]
- Na, K.-H.; Kim, W.-T.; Park, D.-C.; Shin, H.-G.; Lee, S.-H.; Park, J.; Song, T.-H.; Choi, W.-Y. Fabrication and characterization of the magnetic ferrite nanofibers by electrospinning process. Thin Solid Film 2018, 660, 358–364. [Google Scholar] [CrossRef]
- Rajarao, G.K.; Lakshmanan, R.; Okoli, C.; Boutonnet, M.; Ja, S. Microemulsion prepared magnetic nanoparticles for phosphate removal: Time efficient studies. J. Environ. Chem. Eng. 2014, 2, 185–189. [Google Scholar]
- Kekalo, K.; Koo, K.; Zeitchick, E.; Baker, I. Microemulsion Synthesis of Iron Core/Iron Oxide Shell Magnetic Nanoparticles and Their Physicochemical Properties. MRS Proc. 2012, 1416, 9–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion Synthesis of Superparamagnetic Nanoparticles for Bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef]
- Wang, Y.; Nkurikiyimfura, I.; Pan, Z. Sonochemical Synthesis of Magnetic Nanoparticles. Chem. Eng. Commun. 2014, 202, 616–621. [Google Scholar] [CrossRef]
- Fuentes-garc, A.; Alavarse, A.C.; Carolina, A.; Maldonado, M.; Ibarra, M.R.; Fabia, G. Simple Sonochemical Method to Optimize the Heating E ffi ciency of Magnetic Nanoparticles for Magnetic Fluid Hyperthermia. ACS Omega 2020, 5, 26357–26364. [Google Scholar] [CrossRef]
- Holland, H.; Yamaura, M. Synthesis of Magnetite Nanoparticles by Microwave Irradiation and Characterization. In Proceedings of the Conference: International Latin-American Conference on Powder Technology, Atibaia, Brazil, 8–10 November 2009; pp. 434–442. [Google Scholar]
- Aivazoglou, E.; Metaxa, E.; Hristoforou, E. Microwave-assisted synthesis of iron oxide nanoparticles in biocompatible organic environment. AIP Adv. 2018, 8, 048201. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Khan, S.; Khan, S.; Rentschler, S.; Laufer, S.; Deigner, H.-P. Biosynthesis of iron oxide magnetic nanoparticles using clinically isolated Pseudomonas aeruginosa. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Elblbesy, M.A.; Madbouly, A.K.; Hamdan, T.A. Bio-synthesis of magnetite nanoparticles by bacteria. Am. J. Nano Res. Appl. 2014, 2, 98–103. [Google Scholar]
- Balakrishnan, G.S.; Rajendran, K.; Kalirajan, J. Microbial synthesis of magnetite nanoparticles for arsenic removal. J. Appl. Biol. Biotechnol. 2020, 8, 70–75. [Google Scholar]
- Lina, S.; Tejeda-benitez, L.; Hinestroza, J.; Pati, D.; Herrera, A. Green synthesis of iron oxide nanoparticles using Cymbopogon citratus extract and sodium carbonate salt: Nanotoxicological considerations for potential environmental applications. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100377. [Google Scholar]
- Kiwumulo, H.F.; Muwonge, H.; Ibingira, C.; Lubwama, M.; Kirabira, J.B.; Ssekitoleko, R.T. Green synthesis and characterization of iron-oxide nanoparticles using Moringa oleifera: A potential protocol for use in low and middle income countries. BMC Res. Notes 2022, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Aka, T.D.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O.; Ashaduzzaman, M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.; Syhr, C.; Berensmeier, S. Controlled Synthesis of Magnetic Iron Oxide Nanoparticles: Magnetite or Maghemite? Crystals 2020, 10, 214. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Guégan, R.; Popa, C.L.; Motelica-Heino, M.; Ciobanu, C.S.; Predoi, D. Magnetite (Fe3O4) nanoparticles as adsorbents for As and Cu removal. Appl. Clay Sci. 2016, 134, 128–135. [Google Scholar] [CrossRef]
- Klencsár, Z.; Ábrahám, A.; Szabó, L.; Szabó, E.G.; Stichleutner, S.; Kuzmann, E.; Homonnay, Z.; Tolnai, G. The effect of preparation conditions on magnetite nanoparticles obtained via chemical co-precipitation. Mater. Chem. Phys. 2018, 223, 122–132. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Kim, H.; Lee, H.; Ryu, C.; Yoon, J. Synthesis of Magnetic Ferrite Nanoparticles with High Hyperthermia Performance via a Controlled Co-Precipitation Method. Nanomaterials 2019, 9, 1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maity, D.; Ding, J.; Xue, J.-M. Synthesis Of Magnetite Nanoparticles By Thermal Decomposition: Time, Temperature, Surfactant And Solvent Effects. Funct. Mater. Lett. 2008, 1, 189–193. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Panepinto, A.; Socoliuc, V.; Vekas, L.; Muller, R.N.; Laurent, S. Influence of Experimental Parameters of a Continuous Flow Process on the Properties of Very Small Iron Oxide Nanoparticles (VSION) Designed for T1-Weighted Magnetic Resonance Imaging (MRI). Nanomaterials 2020, 10, 757. [Google Scholar] [CrossRef] [Green Version]
- Mourdikoudis, S.; Menelaou, M.; Fiuza-Maneiro, N.; Zheng, G.; Wei, S.; Pérez-Juste, J.; Polavarapu, L.; Sofer, Z. Oleic acid/oleylamine ligand pair: A versatile combination in the synthesis of colloidal nanoparticles. Nanoscale Horiz. 2022, 7, 941–1015. [Google Scholar] [CrossRef] [PubMed]
- Hydrothermal Synthesis Method for Nanoparticle Synthesis—Techinstro. Available online: https://www.techinstro.com/hydrothermal-synthesis-method-for-nanoparticle-synthesis/ (accessed on 23 February 2022).
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal Synthesis of Nanomaterials. J. Nanomater. 2020, 2020, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef] [Green Version]
- Hyun, J.; Osman, I.; Saadullah, G. Magnetite Fe3O4 (111) Surfaces: Impact of Defects on Structure, Stability, and Electronic Properties. Chem. Mater. 2015, 27, 5856–5867. [Google Scholar]
- Richard, B.; Lemyre, J.-L.; Ritcey, A.M. Nanoparticle Size Control in Microemulsion Synthesis. Langmuir 2017, 33, 4748–4757. [Google Scholar] [CrossRef]
- Kimura, K. Magnetic Properties of Magnetite Ultrafine Particles Prepared by W/O Microemulsion Method. Jpn. J. Appl. Phys. 1987, 26, 713. [Google Scholar]
- Gautam, R.K.; Chattopadhyaya, M.C. Functionalized Magnetic Nanoparticles: Adsorbents and Applications BT—Nanomaterials for Wastewater Remediation. In Nanomater. Wastewater Remediat; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 139–159. [Google Scholar]
- Singla, R.; Grieser, F.; Ashokkumar, M. Kinetics and Mechanism for the Sonochemical Degradation of a Nonionic Surfactant. J. Phys. Chem. A 2009, 113, 2865–2872. [Google Scholar] [CrossRef]
- Liu, H.; Ji, S.; Yang, H.; Zhang, H.; Tang, M. Ultrasonic-assisted ultra-rapid synthesis of monodisperse meso-SiO2@Fe3O4 microspheres with enhanced mesoporous structure. Ultrason. Sonochem. 2014, 21, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Perelshtein, I.; Perkas, N.; Gedanken, A. The Sonochemical Functionalization of Textiles; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 161–198. [Google Scholar]
- Choi, J.; Khim, J.; Neppolian, B.; Son, Y. Enhancement of sonochemical oxidation reactions using air sparging in a 36 kHz sonoreactor. Ultrason. Sonochemistry 2018, 51, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Zhu, Y.; Zeng, Y.; Qian, H.; Xiao, J.; Xu, F.; Zhang, L.; Zhao, D. Ultrasonic-Irradiation-Assisted Oriented Assembly of Ordered Monetite Nanosheets Stacking. J. Phys. Chem. B 2009, 113, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Chikan, V.; McLaurin, E.J. Rapid Nanoparticle Synthesis by Magnetic and Microwave Heating. Nanomaterials 2016, 6, 85. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Park, S.-J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [Green Version]
- Kostyukhin, E.; Kustov, L.M. Microwave-assisted synthesis of magnetite nanoparticles possessing superior magnetic properties. Mendeleev Commun. 2018, 28, 559–561. [Google Scholar] [CrossRef]
- Shu, G.; Wang, H.; Zhao, H.-X.; Zhang, X. Microwave-Assisted Synthesis of Black Titanium Monoxide for Synergistic Tumor Phototherapy. ACS Appl. Mater. Interfaces 2018, 11, 3323–3333. [Google Scholar] [CrossRef]
- Strachowski, T.; Grzanka, E.; Mizeracki, J.; Chlanda, A.; Baran, M.; Małek, M.; Niedziałek, M. Microwave-Assisted Hydrothermal Synthesis of Zinc-Aluminum Spinel ZnAl2O4. Materials 2021, 15, 245. [Google Scholar] [CrossRef]
- Eugênia, M.; Brollo, F.; Veintemillas-verdaguer, S.; Salván, C.M.; Morales, P. Key Parameters on the Microwave Assisted Synthesis of Magnetic Nanoparticles for MRI Contrast Agents. Contrast Media Mol. Imaging 2017, 1–13. [Google Scholar]
- Kostyukhin, E.M.; Nissenbaum, V.D.; Abkhalimov, E.V.; Kustov, A.L.; Ershov, B.G.; Kustov, L.M. Microwave-Assisted Synthesis of Water-Dispersible Humate-Coated Magnetite Nanoparticles: Relation of Coating Process Parameters to the Properties of Nanoparticles. Nanomaterials 2020, 10, 1558. [Google Scholar] [CrossRef]
- Schneider, T.; Löwa, A.; Karagiozov, S.; Sprenger, L.; Gutiérrez, L.; Esposito, T.; Marten, G.; Saatchi, K.; Häfeli, U.O. Facile microwave synthesis of uniform magnetic nanoparticles with minimal sample processing. J. Magn. Magn. Mater. 2017, 421, 283–291. [Google Scholar] [CrossRef]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Herranz, F. Microwave-Driven Synthesis of Iron-Oxide Nanoparticles for Molecular Imaging. Molecules 2019, 24, 1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, S.F.; Azman, A.; Pang, S.C. Size Controlled Synthesis of Starch Nanoparticles by a Microemulsion Method. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Roh, Y.; Liu, S.V.; Li, G.; Huang, H.; Phelps, T.J.; Zhou, J. Isolation and Characterization of Metal-Reducing Thermoanaerobacter Strains from Deep Subsurface Environments of the Piceance Basin, Colorado. Appl. Environ. Microbiol. 2002, 68, 6013–6020. [Google Scholar] [CrossRef] [Green Version]
- Batool, F.; Iqbal, M.S.; Khan, S.-U.; Khan, J.; Ahmed, B.; Qadir, M.I. Biologically synthesized iron nanoparticles (FeNPs) from Phoenix dactylifera have anti-bacterial activities. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Gareev, K.G.; Grouzdev, D.S.; Kharitonskii, P.V.; Kosterov, A.; Koziaeva, V.V.; Sergienko, E.S.; Shevtsov, M.A. Magnetotactic Bacteria and Magnetosomes: Basic Properties and Applications. Magnetochemistry 2021, 7, 86. [Google Scholar] [CrossRef]
- Perotti, G.F.; Da Costa, L.P. Biological Materials. In RSC Nanoscience and Nanotechnology; Royal Society of Chemistry: London, UK, 2021; Volume 2021, pp. 316–332. [Google Scholar]
- Vargas, G.; Cypriano, J.; Correa, T.; Leão, P.; Bazylinski, D.A.; Abreu, F. Applications of Magnetotactic Bacteria, Magnetosomes and Magnetosome Crystals in Biotechnology and Nanotechnology: Mini-Review. Molecules 2018, 23, 2438. [Google Scholar] [CrossRef] [Green Version]
- Usov, N.; Gubanova, E. Application of Magnetosomes in Magnetic Hyperthermia. Nanomaterials 2020, 10, 1320. [Google Scholar] [CrossRef]
- Baker, I. Magnetic Nanoparticle Synthesisp; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Kuwano, N.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Lee, K.X. Green Synthesis of Magnetite (Fe3O4) Nanoparticles Using Seaweed (Kappaphycus alvarezii) Extract. Nanoscale Res. Lett. 2016, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. ‘Green’ nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Parajuli, K.; Sah, A.K.; Paudyal, H. Green Synthesis of Magnetite Nanoparticles Using Aqueous Leaves Extracts of Azadirachta indica and Its Application for the Removal of As(V) from Water. Green Sustain. Chem. 2020, 10, 117–132. [Google Scholar] [CrossRef]
- Prasad, C.; Murthy, P.K.; Krishna, R.H.; Rao, R.S.; Suneetha, V.; Venkateswarlu, P. Bio-inspired green synthesis of RGO/Fe3O4 magnetic nanoparticles using Murrayakoenigii leaves extract and its application for removal of Pb(II) from aqueous solution. J. Environ. Chem. Eng. 2017, 5, 4374–4380. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Yee, O.S.; Teow, S.-Y.; Hedayatnasab, Z.; Jahangirian, H.; Webster, T.J.; Kuča, K. Green Synthesis of Fe3O4 Nanoparticles Stabilized by a Garcinia mangostana Fruit Peel Extract for Hyperthermia and Anticancer Activities. Int. J. Nanomed. 2021, 16, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.K.; Gupta, S.; Tyagi, S.; Kumar, M.; Pandiselvam, R.; Daştan, S.D.; Sharifi-Rad, J.; Gola, D.; Arya, A. Green Synthesis of Iron Nanoparticles from Spinach Leaf and Banana Peel Aqueous Extracts and Evaluation of Antibacterial Potential. J. Nanomater. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Nasiri, J.; Rahimi, M.; Hamezadeh, Z.; Motamedi, E.; Naghavi, M.R. Fulfillment of green chemistry for synthesis of silver nanoparticles using root and leaf extracts of Ferula persica: Solid-state route vs. solution-phase method. J. Clean. Prod. 2018, 192, 514–530. [Google Scholar] [CrossRef]
- Pilati, V.; Gomide, G.; Gomes, R.C.; Goya, G.F. Colloidal Stability and Concentration Effects on Nanoparticle Heat Delivery for Magnetic Fluid Hyperthermia. Langmuir 2021, 37, 1129–1140. [Google Scholar] [CrossRef]
- Cortés-Llanos, B.; Ocampo, S.M.; de la Cueva, L.; Calvo, G.F.; Belmonte-Beitia, J.; Pérez, L.; Salas, G.; Ayuso-Sacido, Á. Influence of Coating and Size of Magnetic Nanoparticles on Cellular Uptake for In Vitro MRI. Nanomaterials 2021, 11, 2888. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Dobon, A.; Jorda-Beneyto, M.; Bermudez, J.M. Selection of Nanomaterial-Based Active Agents for Packaging Application: Using Life Cycle Assessment (LCA) as a Tool. Packag. Technol. Sci. 2016, 30, 575–586. [Google Scholar] [CrossRef]
- Bobba, S.; Deorsola, F.A.; Blengini, G.A.; Fino, D. LCA of tungsten disulphide (WS 2 ) nano-particles synthesis: State of art and from-cradle-to-gate LCA. J. Clean. Prod. 2016, 139, 1478–1484. [Google Scholar] [CrossRef]
- Zhang, Z.; Guan, Y.; Xia, T.; Du, J.; Li, T.; Sun, Z.; Guo, C. Influence of exposed magnetic nanoparticles and their application in chemiluminescence immunoassay. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 335–342. [Google Scholar] [CrossRef]
- Dembski, S.; Schneider, C.; Christ, B.; Retter, M. Core-Shell Nanoparticles and Their Use for In Vitro and In Vivo Diagnostics; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ahmadpoor, F.; Masood, A.; Feliu, N.; Parak, W.J.; Shojaosadati, S.A. The Effect of Surface Coating of Iron Oxide Nanoparticles on Magnetic Resonance Imaging Relaxivity. Front. Nanotechnol. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarkistani, M.; Komalla, V.; Kayser, V. Recent Advances in the Use of Iron–Gold Hybrid Nanoparticles for Biomedical Applications. Nanomaterials 2021, 11, 1227. [Google Scholar] [CrossRef]
- Zaloga, J.; Janko, C.; Agarwal, R.; Nowak, J.; Müller, R.; Boccaccini, A.R.; Lee, G.; Odenbach, S.; Lyer, S.; Alexiou, C. Different Storage Conditions Influence Biocompatibility and Physicochemical Properties of Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2015, 16, 9368–9384. [Google Scholar] [CrossRef]
- Widdrat, M.; Kumari, M.; Tompa, É.; Pósfai, M.; Hirt, A.M.; Faivre, D. Keeping Nanoparticles Fully Functional: Long-Term Storage and Alteration of Magnetite. Chem. Plus Chem. 2014, 79, 1225–1233. [Google Scholar] [CrossRef] [Green Version]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [Green Version]
- López-Campos, F.; Candini, D.; Carrasco, E.; Francés, M.A.B.; Candini, D. Nanoparticles applied to cancer immunoregulation. Rep. Pract. Oncol. Radiother. 2019, 24, 47–55. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Kostopoulou, A.; LaGrow, A.P. Magnetic Nanoparticle Composites: Synergistic Effects and Applications. Adv. Sci. 2021, 8, 1–57. [Google Scholar] [CrossRef]
- Singh, G.; Rani, S.; Sharma, G.; Kalra, P.; Singh, N.; Verma, V. Coumarin–derived Organosilatranes: Functionalization at magnetic silica surface and selective recognition of Hg2+ ion. Sens. Actuators B Chem. 2018, 266, 861–872. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Kim, H.-M.; Son, B.S.; Jo, A.; An, J.; Thi, T.A.T.; Nguyen, D.Q.; Jun, B.-H. Silica-Coated Magnetic Iron Oxide Nanoparticles Grafted onto Graphene Oxide for Protein Isolation. Nanomaterials 2020, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Park, J.C.; Gilbert, D.A.; Liu, K.; Louie, A.Y. Supporting information Microwave enhanced silica encapsulation of magnetic nanoparticles. J. Mater. Chem. 2012, 22, 8449–8454. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, N.; Audira, G.; Chen, J.-R.; Siregar, P.; Hsu, H.-S.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. Surface Modification of Magnetic Nanoparticles by Carbon-Coating Can Increase Its Biosafety: Evidences from Biochemical and Neurobehavioral Tests in Zebrafish. Molecules 2020, 25, 2256. [Google Scholar] [CrossRef]
- Baykal, A.; Senel, M.; Unal, B.; Karaoğlu, E.; Sözeri, H.; Toprak, M. Acid Functionalized Multiwall Carbon Nanotube/Magnetite (MWCNT)-COOH/Fe3O4 Hybrid: Synthesis, Characterization and Conductivity Evaluation. J. Inorg. Organomet. Polym. Mater. 2013, 23, 726–735. [Google Scholar] [CrossRef]
- Moreno-Bárcenas, A.; Zapata, J.A.A.; Alcalá, M.E.; Ramírez, J.T.; Hernández, A.M.; García-García, A. Evolution of Nanostructured Carbon Coatings Quality via RT-CVD and Their Tribological Behavior on Nodular Cast Iron. Metals 2022, 12, 517. [Google Scholar] [CrossRef]
- Kyesmen, P.I.; Nombona, N.; Diale, M. A Promising Three-Step Heat Treatment Process for Preparing CuO Films for Photocatalytic Hydrogen Evolution from Water. ACS Omega 2021, 6, 33398–33408. [Google Scholar] [CrossRef]
- Chen, Z.; Dai, X.J.; Lamb, P.R.; du Plessis, J.; Leal, D.R.D.C.; Magniez, K.; Fox, B.L.; Wang, X. Coating and Functionalization of Carbon Fibres Using a Three-Step Plasma Treatment. Plasma Process. Polym. 2013, 10, 1100–1109. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. Cryst. Eng. Comm. 2017, 19, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, L.M.; Alvarez, V.A. Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices. Bioengineering 2019, 6, 75. [Google Scholar] [CrossRef]
- Ortega, G.; Reguera, E. Biomedical Applications of Magnetite Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Shiri, M.S.Z.; Henderson, W.; Mucalo, M.R. A Review of The Lesser-Studied Microemulsion-Based Synthesis Methodologies Used for Preparing Nanoparticle Systems of The Noble Metals, Os, Re, Ir and Rh. Materials 2019, 12, 1896. [Google Scholar] [CrossRef] [Green Version]
- Slimani, S.; Concas, G.; Congiu, F.; Barucca, G.; Yaacoub, N.; Talone, A.; Smari, M.; Dhahri, E.; Peddis, D.; Muscas, G. Hybrid Spinel Iron Oxide Nanoarchitecture Combining Crystalline and Amorphous Parent Material. J. Phys. Chem. C 2021, 125, 10611–10620. [Google Scholar] [CrossRef]
- Mylkie, K.; Nowak, P.; Rybczynski, P.; Ziegler-Borowska, M. Polymer-Coated Magnetite Nanoparticles for Protein Immobilization. Materials 2021, 14, 248. [Google Scholar] [CrossRef]
- Smit, M.; Lutz, M. Polymer-coated magnetic nanoparticles for the efficient capture of Mycobacterium tuberculosis (Mtb). SN Appl. Sci. 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Mirshahghassemi, S.; Cai, B.; Lead, J.R. Evaluation of polymer-coated magnetic nanoparticles for oil separation under environmentally relevant conditions: Effect of ionic strength and natural organic macromolecules. Environ. Sci. Nano 2016, 3, 780–787. [Google Scholar] [CrossRef]
- Kim, D.; Yu, M.K.; Lee, T.S.; Park, J.J.; Jeong, Y.Y.; Jon, S. Amphiphilic polymer-coated hybrid nanoparticles as CT/MRI dual contrast agents. Nanotechnology 2011, 22, 155101. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Liu, Q.-H.; Li, K.; Li, Y.; Chang, N. Magnetic iron(III)-based framework composites for the magnetic solid-phase extraction of fungicides from environmental water samples. J. Sep. Sci. 2017, 41, 1129–1137. [Google Scholar] [CrossRef]
- Sommertune, J.; Sugunan, A.; Ahniyaz, A.; Bejhed, R.S.; Fornara, A. Polymer / Iron Oxide Nanoparticle Composites—A Straight Forward and Scalable Synthesis Approach. Int. J. Mol. Sci. 2015, 16, 19752–19768. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, N.; Huang, X.; Li, F.; Davis, T.P.; Qiao, R.; Ling, D. Polymer-Assisted Magnetic Nanoparticle Assemblies for Biomedical Applications. ACS Appl. Bio Mater. 2019, 3, 121–142. [Google Scholar] [CrossRef] [Green Version]
- Beyou, E.; Bourgeat-Lami, E. Organic–inorganic hybrid functional materials by nitroxide-mediated polymerization. Prog. Polym. Sci. 2021, 121, 101434. [Google Scholar] [CrossRef]
- Behrens, S.; Appel, I. Magnetic nanocomposites. Curr. Opin. Biotechnol. 2016, 39, 89–96. [Google Scholar] [CrossRef]
- Demirelli, M.; Karaoglu, E.; Baykal, A.; Sozeri, H. M-hexaferrite–APTES/Pd(0) Magnetically Recyclable Nano Catalysts (MRCs). J. Inorg. Organomet. Polym. Mater. 2013, 23, 1274–1281. [Google Scholar] [CrossRef]
- Karaoglu, E.; Baykal, A. CoFe2O4–Pd (0) Nanocomposite: Magnetically Recyclable Catalyst. J. Supercond. Nov. Magn. 2014, 27, 2041–2047. [Google Scholar] [CrossRef]
- Junejo, Y.; Baykal, A.; Sözeri, H. Simple hydrothermal synthesis of Fe3O4-PEG nanocomposite. Open Chem. 2013, 11, 1527–1532. [Google Scholar] [CrossRef]
- Watt, J.; Collins, A.M.; Vreeland, E.C.; Montaño, G.A.; Huber, D.L. Magnetic Nanocomposites and Their Incorporation into Higher Order Biosynthetic Functional Architectures. ACS Omega 2018, 3, 503–508. [Google Scholar] [CrossRef]
- Alveroǧlu, E.; Sözeri, H.; Baykal, A.; Kurtan, U.; Şenel, M. Fluorescence and magnetic properties of hydrogels containing Fe3O4 nanoparticles. J. Mol. Struct. 2013, 1037, 361–366. [Google Scholar] [CrossRef]
- Demir, A.; Baykal, A.; Sözeri, H.; Topkaya, R. Low temperature magnetic investigation of Fe3O4 nanoparticles filled into multiwalled carbon nanotubes. Synth. Met. 2014, 187, 75–80. [Google Scholar] [CrossRef]
- Akal, Z.; Alpsoy, L.; Baykal, A. Biomedical applications of SPION@APTES@PEG-folic acid@carboxylated quercetin nanodrug on various cancer cells. Appl. Surf. Sci. 2016, 378, 572–581. [Google Scholar] [CrossRef]
- Hulla, J.E.; Sahu, S.C.; Hayes, A.W. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef] [Green Version]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef]
- Hose, R.C. Prof. Richard Zsigmondy. Nature 1929, 124, 845–846. [Google Scholar]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [Green Version]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P. Gold-iron oxide nanohybrids: Insights into colloidal stability and surface-enhanced Raman detection. Nanoscale Adv. 2021, 3, 6438–6445. [Google Scholar] [CrossRef]
- Kah, J.; Yeo, E.; He, S.; Engudar, G. Gold Nanorods in Photomedicine in Applications of Nanoscience in Photomedicine; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 221–248. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef]
- Vemulkar, T.; Mansell, R.; Petit, D.C.M.C.; Cowburn, R.P.; Lesniak, M.S. Highly tunable perpendicularly magnetized synthetic antiferromagnets for biotechnology applications. Appl. Phys. Lett. 2015, 107, 012403. [Google Scholar] [CrossRef] [Green Version]
- Panahi, H.A.; Alaei, H.S. β-Cyclodextrin/thermosensitive containing polymer brushes grafted onto magnetite nano-particles for extraction and determination of venlafaxine in biological and pharmaceutical samples. Int. J. Pharm. 2014, 476, 178–184. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Zhang, J.; Dong, W. Design of a pH-sensitive magnetic composite hydrogel based on salecan graft copolymer and Fe3O4@SiO2nanoparticles as drug carrier. Int. J. Biol. Macromol. 2018, 107, 1811–1820. [Google Scholar] [CrossRef]
- Otero-Lorenzo, R.; Dávila-Ibáñez, A.B.; Comesaña-Hermo, M.; Correa-Duarte, M.A.; Salgueiriño, V. Synergy effects of magnetic silica nanostructures for drug delivery applications. J. Mater. Chem. B 2014, 2, 2645–2653. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Biodistribution and targeting properties of iron oxide nanoparticles for treatments of cancer and iron anemia disease. Nanotoxicology 2019, 13, 573–596. [Google Scholar] [CrossRef] [PubMed]
- Del Sol-Fernández, S.; Portilla-Tundidor, Y.; Gutiérrez, L.; Odio, O.F.; Reguera, E.; Barber, D.F.; Morales, M.P. Flower-like Mn-Doped Magnetic Nanoparticles Functionalized with αvβ3-Integrin-Ligand to Efficiently Induce Intracellular Heat after Alternating Magnetic Field Exposition, Triggering Glioma Cell Death. ACS Appl. Mater. Interfaces 2019, 11, 26648–26663. [Google Scholar] [CrossRef]
- Xu, K.; Yao, H.; Hu, J.; Zhou, J.; Zhou, L.; Wei, S. Pre-drug Self-assembled Nanoparticles: Recovering activity and overcoming glutathione-associated cell antioxidant resistance against photodynamic therapy. Free Radic. Biol. Med. 2018, 124, 431–446. [Google Scholar] [CrossRef]
- Berry, C.C.; Wells, S.; Charles, S.; Curtis, A.S. Dextran and albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials 2003, 24, 4551–4557. [Google Scholar] [CrossRef]
- Gupta, A.K.; Curtis, A.S. Lactoferrin and ceruloplasmin derivatized superparamagnetic iron oxide nanoparticles for targeting cell surface receptors. Biomaterials 2003, 25, 3029–3040. [Google Scholar] [CrossRef]
- Pöttler, M.; Fliedner, A.; Schreiber, E.; Janko, C.; Friedrich, R.P.; Bohr, C.; Döllinger, M.; Alexiou, C.; Dürr, S. Impact of Superparamagnetic Iron Oxide Nanoparticles on Vocal Fold Fibroblasts: Cell Behavior and Cellular Iron Kinetics. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ramchandran, V.; Gernand, J.M. Examining the in vivo pulmonary toxicity of engineered metal oxide nanomaterials using a genetic algorithm-based dose-response-recovery clustering model. Comput. Toxicol. 2019, 13, 100113. [Google Scholar] [CrossRef]
- Sadeghi, L.; Babadi, V.Y.; Espanani, H.R. Toxic effects of the Fe2O3 nanoparticles on the liver and lung tissue. Bratisl. Med. J. 2015, 116, 373–378. [Google Scholar] [CrossRef]
- Parivar, K.; Fard, F.M.; Bayat, M.; Alavian, S.M.; Motavaf, M. Evaluation of Iron Oxide Nanoparticles Toxicity on Liver Cells of BALB/c Rats. Iran. Red Crescent Med. J. 2016, 18, e28939. [Google Scholar] [CrossRef] [Green Version]
- Osman, N.M.; Sexton, D.; Saleem, I.Y. Toxicological assessment of nanoparticle interactions with the pulmonary system. Nanotoxicology 2019, 14, 21–58. [Google Scholar] [CrossRef] [PubMed]
- Omidkhoda, A.; Mozdarani, H.; Movasaghpoor, A.; Pour Fatholah, A.A. Study of apoptosis in labeled mesenchymal stem cells with superparamagnetic iron oxide using neutral comet assay. Toxicol. Vitr. 2007, 21, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Z.; Lv, H.; Wu, L.; Cui, Y.; Yao, H.; Li, J.; Zhang, H.; Yang, B.; Jiang, J. Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites. Int. J. Nanomed. 2019, 14, 573–589. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.-M.; Hsiao, J.-K.; Chen, Y.-C.; Chien, L.-Y.; Yao, M.; Chen, Y.-K.; Ko, B.-S.; Hsu, S.-C.; Tai, L.-A.; Cheng, H.-Y.; et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Balas, M.; Din, I.P.; Hermenean, A.; Cinteza, L.; Dinischiotu, A. Exposure to Iron Oxide Nanoparticles Coated with Phospholipid-Based Polymeric Micelles Induces Renal Transitory Biochemical and Histopathological Changes in Mice. Materials 2021, 14, 2605. [Google Scholar] [CrossRef]
- Hataminia, F.; Noroozi, Z.; Eslam, H.M. Investigation of iron oxide nanoparticle cytotoxicity in relation to kidney cells: A mathematical modeling of data mining. Toxicol. Vitr. 2019, 59, 197–203. [Google Scholar] [CrossRef]
- Serkova, N.J.; Renner, B.; Larsen, B.A.; Stoldt, C.R.; Hasebroock, K.M.; Bradshaw-Pierce, E.L.; Holers, V.M.; Thurman, J.M. Renal Inflammation: Targeted Iron Oxide Nanoparticles for Molecular MR Imaging in Mice. Radiology 2010, 255, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Cao, S.; Liang, S.; Tan, C.H.; Luo, B.; Xu, X.; Saw, P.E. Differently Charged Super-Paramagnetic Iron Oxide Nanoparticles Preferentially Induced M1-Like Phenotype of Macrophages. Front. Bioeng. Biotechnol. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Gu, Z.; Liu, T.; Tang, J.; Yang, Y.; Song, H.; Tuong, Z.K.; Fu, J.; Yu, C. Mechanism of Iron Oxide-Induced Macrophage Activation: The Impact of Composition and the Underlying Signaling Pathway. J. Am. Chem. Soc. 2019, 141, 6122–6126. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Liu, Z.W.; Allaker, R.P.; Reip, P.; Oxford, J.; Ahmad, Z.; Ren, G. A review of nanoparticle functionality and toxicity on the central nervous system. Nanotechnol. Brain Future 2013, 7, 313–332. [Google Scholar]
- Hajsalimi, G.; Taheri, S.; Shahi, F.; Attar, F.; Ahmadi, H.; Falahati, M. Interaction of iron nanoparticles with nervous system: An in vitro study. J. Biomol. Struct. Dyn. 2017, 36, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Apopa, P.L.; Qian, Y.; Shao, R.; Guo, N.L.; Schwegler-Berry, D.; Pacurari, M.; Porter, D.; Shi, X.; Vallyathan, V.; Castranova, V.; et al. Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Part. Fibre Toxicol. 2009, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.; Du, J.; Jin, R.; Zhu, W.; Liu, L.; Yang, L.; Li, M.; Gong, Q.; Song, B.; Anderson, J.M.; et al. Iron oxide nanoparticles promote vascular endothelial cells survival from oxidative stress by enhancement of autophagy. Regen. Biomater. 2019, 6, 221–229. [Google Scholar] [CrossRef]
- Wen, T.; Du, L.; Chen, B.; Yan, D.; Yang, A.; Liu, J.; Gu, N.; Meng, J.; Xu, H. Iron oxide nanoparticles induce reversible endothelial-to-mesenchymal transition in vascular endothelial cells at acutely non-cytotoxic concentrations. Part. Fibre Toxicol. 2019, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, A.; Cañete, M.; Roca, A.G.; Calero, M.; Veintemillas-Verdaguer, S.; Serna, C.J.; Morales, M.D.P.; Miranda, R. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology 2009, 20, 115103. [Google Scholar] [CrossRef]
- Yang, J.-X.; Tang, W.-L.; Wang, X.-X. Superparamagnetic iron oxide nanoparticles may affect endothelial progenitor cell migration ability and adhesion capacity. Cytotherapy 2010, 12, 251–259. [Google Scholar] [CrossRef]
- Cochran, D.B.; Wattamwar, P.P.; Wydra, R.; Hilt, J.Z.; Anderson, K.W.; Eitel, R.E.; Dziubla, T.D. Suppressing iron oxide nanoparticle toxicity by vascular targeted antioxidant polymer nanoparticles. Biomater. 2013, 34, 9615–9622. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the In Vitro and In Vivo Toxicity of Superparamagnetic Iron Oxide Nanoparticles. Chem. Rev. 2011, 112, 2323–2338. [Google Scholar] [CrossRef] [Green Version]
- Schimpel, C.; Resch, S.; Flament, G.; Carlander, D.; Vaquero, C.; Bustero, I.; Falk, A. A methodology on how to create a real-life relevant risk profile for a given nanomaterial. ACS Chem. Health Saf. 2018, 25, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Sarpong-Kumankomah, S.; Gibson, M.A.; Gailer, J. Organ damage by toxic metals is critically determined by the bloodstream. Co-Ord. Chem. Rev. 2018, 374, 376–386. [Google Scholar] [CrossRef]
- Krug, H.F. Nanosafety Research-Are We on the Right Track? Angew. Chem. Int. Ed. Engl. 2014, 53, 12304–12319. [Google Scholar] [CrossRef] [PubMed]
- Motayagheni, N. Modified Langendorff technique for mouse heart cannulation: Improved heart quality and decreased risk of ischemia. MethodsX 2017, 4, 508–512. [Google Scholar] [CrossRef]
- Tipton, C.M.; Matthes, R.D.; Tcheng, T.; Dowell, R.T.; Vailas, A.C. The use of the Langendorff preparation to study the bradycardia of training. Med. Sci. Sport. 1977, 9, 220–230. [Google Scholar]
- Bell, R.M.; Mocanu, M.M.; Yellon, D.M. Retrograde heart perfusion: The Langendorff technique of isolated heart perfusion. J. Mol. Cell Cardiol. 2011, 50, 940–950. [Google Scholar] [CrossRef]
- Zimmer, H.-G. The Isolated Perfused Heart and Its Pioneers. Physiology 1998, 13, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Stone, V.; Johnston, H.; Schins, R.P.F. Development of in vitro systems for nanotoxicology: Methodological considerations in vitro methods for nanotoxicology Vicki Stone et al. Crit. Rev. Toxicol. 2009, 39, 613–626. [Google Scholar] [CrossRef]
- Erofeev, A.; Gorelkin, P.; Garanina, A.; Alova, A.; Efremova, M.; Vorobyeva, N.; Edwards, C.; Korchev, Y.; Majouga, A. Novel method for rapid toxicity screening of magnetic nanoparticles. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yuen, H.-W.; Becker, W. Iron Toxicity; StatPearls Publishing: Florida, FL, USA, 2019. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.; Lerner, E. Nitric oxide inhibition strategies. Futur. Sci. OA 2015, 1, 1. [Google Scholar] [CrossRef]
- Li, Q.; Yon, J.-Y.; Cai, H. Mechanisms and Consequences of eNOS Dysfunction in Hypertension. J. Hypertens. 2015, 33, 1128–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Skrzypiec-Spring, M.; Grotthus, B.; Szeląg, A.; Schulz, R. Isolated heart perfusion according to Langendorff—Still viable in the new millennium. J. Pharmacol. Toxicol. Methods 2007, 55, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Manuel, A.R.-L.; Martinez-Cuevas, P.P.; Rosas-Hernandez, H.; Oros-Ovalle, C.; Bravo-Sanchez, M.; Martinez-Castañon, G.A.; Gonzalez, C. Evaluation of vascular tone and cardiac contractility in response to silver nanoparticles, using Langendorff rat heart preparation. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1507–1518. [Google Scholar] [CrossRef]
- Vargas, J.R.; Harald, O.; Carmen, N.B.; Karen, G. Magnetic nanoparticle behavior evaluation on cardiac tissue contractility through Langendorff rat heart technique as a nanotoxicology parameter. Appl. Nanosci. 2021, 11, 2383–2396. [Google Scholar] [CrossRef]
- Thorat, N.D.; Otari, S.V.; Patil, R.M.; Bohara, R.A.; Yadav, H.M.; Koli, V.B.; Chaurasia, A.K.; Ningthoujam, R.S. Synthesis, Characterization and Biocompatibility of Chitosan functionalized superparamagnetic nanoparticles for heat activated curing of cancer cells Published. Dalton Trans. 2014, 43, 17343–17351. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, 465–469. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar]
- Spirou, S.V.; Lima, S.A.C.; Bouziotis, P.; Vranješ-Djurić, S.; Efthimiadou, E.; Laurenzana, A.; Barbosa, A.I.; Garcia-Alonso, I.; Jones, C.; Jankovic, D.; et al. Recommendations for In Vitro and In Vivo Testing of Magnetic Nanoparticle Hyperthermia Combined with Radiation Therapy. Nanomaterials 2018, 8, 306. [Google Scholar] [CrossRef] [Green Version]
- Jespersen, B.; Tykocki, N.R.; Watts, S.W.; Cobbett, P.J. Measurement of smooth muscle function in the isolated tissue bath-applications to pharmacology research. J. Vis. Exp. 2015, 95, e52324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, C.; Corbacho, A.M.; Eiserich, J.P.; Garcia, C.; Lopez-Barrera, F.; Morales-Tlalpan, V.; Barajas-Espinosa, A.; Diaz-Muñoz, M.; Rubio, R.; Lin, S.-H.; et al. 16K-Prolactin Inhibits Activation of Endothelial Nitric Oxide Synthase, Intracellular Calcium Mobilization, and Endothelium-Dependent Vasorelaxation. Endocrinology 2004, 145, 5714–5722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachler, G.; von Goetz, N.; Hungerbühler, K. A physiologically based pharmacokinetic model for ionic silver and silver nanoparticles. Int. J. Nanomed. 2013, 8, 3365–3382. [Google Scholar]
- Al-Jamal, K.T.; Bai, J.; Wang, J.T.-W.; Protti, A.; Southern, P.; Bogart, L.; Heidari, H.; Li, X.; Cakebread, A.; Asker, D.; et al. Magnetic Drug Targeting: Preclinical in Vivo Studies, Mathematical Modeling, and Extrapolation to Humans. Nano Lett. 2016, 16, 5652–5660. [Google Scholar] [CrossRef] [Green Version]
- Corá, L.; Romeiro, F.; Stelzer, M.; Américo, M.; Oliveira, R.; Baffa, O.; Miranda, J. AC biosusceptometry in the study of drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1223–1241. [Google Scholar] [CrossRef]
- Prospero, A.G.; Fidelis-De-Oliveira, P.; Soares, G.A.; Miranda, M.F.; Pinto, L.A.; Dos Santos, D.C.; Silva, V.D.S.; Zufelato, N.; Bakuzis, A.F.; Miranda, J.R. AC biosusceptometry and magnetic nanoparticles to assess doxorubicin-induced kidney injury in rats. Nanomedicine 2020, 15, 511–525. [Google Scholar] [CrossRef]
- Alphandéry, E. Bio-synthesized iron oxide nanoparticles for cancer treatment. Int. J. Pharm. 2020, 586, 119472. [Google Scholar] [CrossRef]
- Wei, H.; Hu, Y. Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int. J. Nanomed. 2021, 16, 6097. [Google Scholar] [CrossRef]
- Khan, L.U.; Petry, R.; Paula, A.J.; Knobel, M.; Ste, D. Protein Corona Formation on Magnetic Nanoparticles Conjugated with Luminescent Europium Complexes. ChemNanoMat 2018, 4, 1202–1208. [Google Scholar] [CrossRef]
- Nedyalkova, M.; Donkova, B.; Romanova, J.; Tzvetkov, G.; Madurga, S.; Simeonov, V. Iron oxide nanoparticles—In vivo/in vitro biomedical applications and in silico studies. Adv. Colloid Interface Sci. 2017, 249, 192–212. [Google Scholar] [CrossRef] [Green Version]
- Kostal, J. Computational Chemistry in Predictive Toxicology: Status Quo et Quo Vadis? 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 10. [Google Scholar]
- Antonelli, A.; Sfara, C.; Weber, O.; Pison, U.; Manuali, E.; Salamida, S.; Magnani, M. Characterization of ferucarbotran-loaded RBCs as long circulating magnetic contrast agents. Nanomedicine 2016, 11, 2781–2795. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Nanoreg Data Logging Templates for the Environmental, Health and Safety Assessment of Nanomaterials; European Commission: Luxembourg, 2013. [Google Scholar]
- European Union. Regulation (Eu) No 1169/2011 of the European Parliament; European Union: Luxembourg, 2011. [Google Scholar]
- ISO 14577-1:2015; Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters–Part 1: Test Method. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14577:-1:ed-2:v1:en (accessed on 24 August 2022).
- National Science and Technology Council Committee on Technology. National Nanotechnology Initiative: Strategic Plan National Science and Technology Council Subcommittee on Nanoscale Science, Engineering, and Technology Committee on Technology About the National Science and Technology Council; National Science and Technology Council Committee on Technology: Washington, DC, USA, 2014. [Google Scholar]
- FDA. Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Biotechnol. Law Rep. 2011, 30, 613–616. [Google Scholar] [CrossRef]
- ECHA. Appendix R7-1 for Nanoforms Applicable to Chapter R7a Endpoint Specific Guidance; ECHA: Helsinki, Finland, 2021. [Google Scholar]
- Canadian Enviromental Protection Act. Framework for the Risk Assessment of Manufactured Nanomaterials under the Canadian Environmental Protection Act, 1999 Draft Environment and Climate Change Canada Health Canada Draft June 2022 Executive Summary; Canadian Enviromental Protection Act: Victoria, BC, Canada, 2022. [Google Scholar]
- European Chemicals Agency. Understanding REACH—ECHA. 2018. Available online: https://echa.europa.eu/regulations/reach/understanding-reach (accessed on 26 September 2018).
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Kodali, V.; Gaffrey, M.; Wang, W.; Minard, K.R.; Karin, N.J.; Teeguarden, J.G.; Thrall, B.D. Iron oxide nanoparticle agglomeration influences dose rates and modulates oxidative stress-mediated dose-response profiles in vitro. Nanotoxicology 2014, 8, 663–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Lowry, G.V. Progress towards standardized and validated characterizations for measuring physicochemical properties of manufactured nanomaterials relevant to nano health and safety risks. NanoImpact 2018, 9, 14–30. [Google Scholar] [CrossRef]
- Barik, B.K.; Mishra, M. Nanoparticles as a potential teratogen: A lesson learnt from fruit fly. Nanotoxicology 2018, 13, 258–284. [Google Scholar] [CrossRef]
- Yu, X.; Hong, F.; Zhang, Y.-Q. Bio-effect of nanoparticles in the cardiovascular system. J. Biomed. Mater. Res. Part A 2016, 104, 2881–2897. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Dawoud, A.; Helal, M.A. Interaction of nanoparticles with biological macromolecules: A review of molecular docking studies. Nanotoxicology 2020, 15, 66–95. [Google Scholar] [CrossRef]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2015, 2, 43–70. [Google Scholar] [CrossRef]






| Synthesis Route | Temperature (°C) | Environment | Time | Size Control | Shape Control | Efficiency Output | Magnetite (XRD Pattern) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Aqueous routes | Coprecipitation | <100 | Insert atmosphere | Minutes | Relatively broad | Bad | High | Precursor-dependent | [92,93,94] |
| Thermal decomposition | 100–300 | Insert atmosphere | Hours to days | Excellent | Excellent | High | Oxygen-dependent | [95,96,97] | |
| Hydrothermal | 150–200 | High pressure | Hours to days | Excellent | Excellent | High | Temperature-dependent | [98] | |
| Sol–gel | 100–300 | Ambient | Hours | Good | Good | Medium | Poor magnetite presence | [99,100,101,102] | |
| Microemulsion | <100 | Ambient | Hours | Good | Good | Low | Poor magnetite presence | [103,104,105] | |
| Assisted routes | Sonochemical assisted | <50 | Ambient | Minutes | Good | Bad | Medium | Cavitation- and frequency-dependent | [106,107] |
| Microwaved assisted | 100–200 | Ambient | Minutes to hours | Medium | Good | Medium | High magnetite presence | [108,109] | |
| Biologic routes | Bacteria driven | Room temp. | Ambient | Hours to days | Broad | Bad | Low | Biologic-assistant-dependent | [110,111,112] |
| Green | Room temp. | Ambient | Minutes | Relatively good | Good | Low | Medium magnetite presence. Leaves nature-dependent | [113,114,115] | |
| Tissue | Concentration | Morphology | Size | Coating | Methodology | Effect | Ref. |
|---|---|---|---|---|---|---|---|
| Fibroblast (hTERT human) | 0.1–0.02 mg/mL | Spherical | 7.8–9.6 nm | Dextran, albumin Lactoferrin, ceruloplasmin | BrdU assay | Cellular death | [222,223,224] |
| Lung cells (A549) | 20–40 mg/kg | Spherical | 20–107.7 nm | Bare | TB staining, ROS, Comet | Enhancement of free radicals and reduction in the GSH DNA oxidative injuries (Comet) Low—no toxicity (TB, ROS) Increased TP and LDH Non-biomechanical damage Cell Young’s modulus decreased (25–28%) | [225,226] |
| Liver rat cell (BAL3A rat) | 20–40 mg/kg 25, 50, 75, 150, 300 µg/g | Spherical | 107.7 nm 20–30 nm | Bare Liposomes PEG | MTT, LDH | Nontoxic below 75 µgmL−1 Nonalcoholic fatty liver disease (NAFLD) inflammation LDH leaking Iron overload affected by NAFLD | [226,227,228] |
| Mesenchymal mother cell (MSC human) | 50, 100, 250 mg/mL 25, 50, 100, 150 µg/mL | Spherical | 80–150 nm 48 nm | Protamine sulfate PDA | Comet | No significant effect Increased proliferation index and migration ability | [229,230,231] |
| Kidney cells (Cos-7 monkey) | 15 mg/kg 1–100 µg/mL | Spheric-like Ferrofluid | 13–122 nm 9.7 nm | Phospholipid-based polymeric micelles DOX | MTT, MTS | Cell viability reduced Particle charge (+)-induced high cytotoxicity Oxidative stress, reverted by tissue | [232,233,234] |
| Macrophage (human) | 2.73 mg/mL | Ferrofluid Ellipsoidal | 320–490 nm | Bare SiO2 | MTS, BrdU assay | Time-dependent cell viability (7 days, 20%) Induced M1 activation | [235,236] |
| Nervous system cells (human, PC12) | 0–1000 µg/mL | Spherical Hollow spheres | 5–100 nm | PGA SiO2 Dextran Bare | MTS, LDH | MTS increased production DNA fragmentation, apoptotic Conformational changes in Tau protein Oxidative stress | [237,238,239] |
| Endothelial cells (BAECs, HUVECs) | 50 µg/mL 0–100 µg/mL 0, 300, 600 µg/mL | Spherical Spherical–like | 50–600 nm 5–10 nm 10 nm | Bare Dextran PSC | PI staining, Redox | Cellular intake Cell viability 80 ± 3% Promotes cell survival by autophagy Peroxidase-like activity | [240,241,242] |
| Cancer (multiple) | 50, 100, 500 µg/mL | Spherical–like | 6 nm | DMSA | MTT | High cell viability >90% Trigger immune response High ROS activity | [240,241,242,243] |
| Vascular system (A10 rat) | 50, 100, 200, 400 µg/mL | Spherical–like | 150–160 nm | Bare Citric acid | Redox, MTT | Decreased cell viability Increased actin and calponin expression Concentration-dependent toxicity Migration of EPC reduced | [242,244,245] |
| Biological Studies | Type of Assay | Assays in MNPs | Ref. |
|---|---|---|---|
| In vitro | Suspension (HL60, K562) Monolayers (MCF-7, U87MG) Cultured | CCK8, MTT, TB, LDH, Comet | [268,278] |
| Ex vivo | Langendorff isolated system, in silico studies | Perfusion pressure, protein expression, mediator count, liver, spleen, lungs, heart | [250,264,279] |
| In vivo | Biodistribution, histological staining | VIP, liver, spleen, lungs, heart | [268,280] |
| Organization | Nanomaterial Definition * | Status | Last Meeting/Proposal | Ref. |
|---|---|---|---|---|
| NANoREG (European Union, EU, European Commission, EC) | Taken from EC: “Any intentionally produced material that has one or more dimensions of the order of 100 nm or less or that is composed of discrete functional parts, either internally or at the surface, many of which have one or more dimensions of the order of 100 nm or less”. | Toxicological data gathering | 2014, updated by NanoFATE in 2022 | [281,282] |
| International Organization for Standardization (ISO) | “Any material with any external dimension in the nanoscale or having an internal structure or surface in the nanoscale”. | Terms and vocabulary for nano-objects | 2017 | [283] |
| FDA (United States of America, National Nanotechnology Initiative, NNI) | Taken from the NNI: “The understanding and control of matter at dimensions between approximately 1 and 100 nanometers, where unique phenomena enable novel applications”. | Nonbinding recommendations for manufacturers | 2014 | [284,285] |
| ECHA (European Union) | “A natural, incidental, or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in size range 1 nm–100 nm”. | Guidance for terms, vocabulary, and sample dispersion and aggregation of nanoforms | Draft 2021 | [286] |
| CEPA (Canada) | “Any manufactured substance or product, as well as any component material, ingredient, device, or structure, if it has at least one external dimension that is at or within the nanoscale, or if it has internal or surface structure that is at the nanoscale, or if it has all dimensions that are smaller or larger than the nanoscale and exhibits at least one nanoscale property or phenomenon”. | Guidance framework for adapting nanomaterials to existing practices | Draft 2022 | [287] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Ortiz, J.R.; Gonzalez, C.; Esquivel, K. Magnetic Iron Nanoparticles: Synthesis, Surface Enhancements, and Biological Challenges. Processes 2022, 10, 2282. https://doi.org/10.3390/pr10112282
Vargas-Ortiz JR, Gonzalez C, Esquivel K. Magnetic Iron Nanoparticles: Synthesis, Surface Enhancements, and Biological Challenges. Processes. 2022; 10(11):2282. https://doi.org/10.3390/pr10112282
Chicago/Turabian StyleVargas-Ortiz, Jesús Roberto, Carmen Gonzalez, and Karen Esquivel. 2022. "Magnetic Iron Nanoparticles: Synthesis, Surface Enhancements, and Biological Challenges" Processes 10, no. 11: 2282. https://doi.org/10.3390/pr10112282
APA StyleVargas-Ortiz, J. R., Gonzalez, C., & Esquivel, K. (2022). Magnetic Iron Nanoparticles: Synthesis, Surface Enhancements, and Biological Challenges. Processes, 10(11), 2282. https://doi.org/10.3390/pr10112282