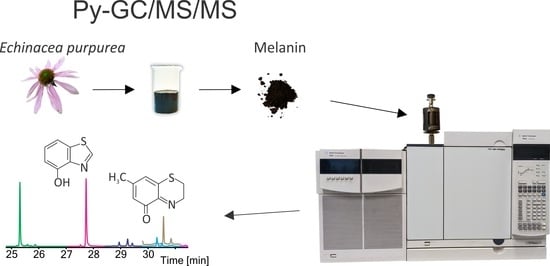
Are Plants Capable of Pheomelanin Synthesis? Gas Chromatography/Tandem Mass Spectrometry Characterization of Thermally Degraded Melanin Isolated from Echinacea purpurea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Compounds
2.2. Preparation of Melanin Standard
2.3. Isolation of Melanin from Echinacea purpurea
2.4. Conditions of Py-GC/MS and Py-GC/MS/MS Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [PubMed] [Green Version]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef] [Green Version]
- Coelho, J.; Barros, L.; Dias, M.I.; Finimundy, T.C.; Amaral, J.S.; Alves, M.J.; Calhelha, R.C.; Santos, P.F.; Ferreira, I.C.F.R. Echinacea purpurea (L.) Moench: Chemical Characterization and Bioactivity of Its Extracts and Fractions. Pharmaceuticals 2020, 13, 125. [Google Scholar] [CrossRef]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, B. Medicinal properties of Echinacea: A critical review. Phytomedicine 2003, 10, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Karg, C.A.; Wang, P.; Vollmar, A.M.; Moser, S. Re-opening the stage for Echinacea research—Characterization of phylloxanthobilins as a novel anti-oxidative compound class in Echinacea purpurea. Phytomedicine 2019, 60, 152969. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 1–28. [Google Scholar] [CrossRef] [Green Version]
- d’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stępień, K.; Dzierżęga-Lecznar, A.; Tam, I.; Kurkiewicz, S. Structure and biological activity of natural melanin pigments. In Chemistry and Pharmacology of Naturally Occuring Bioactive Compounds, 1st ed.; Brahmachari, G., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 211–237. [Google Scholar]
- Singh, S.; Nimse, S.B.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Gajjar, A.; Ghadge, V.A.; Kumar, P.; Shinde, P.B. Microbial melanin: Recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol. Adv. 2021, 53, 107773. [Google Scholar] [CrossRef] [PubMed]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef]
- Pugh, N.D.; Balachandran, P.; Lata, H.; Dayan, F.E.; Joshi, V.; Bedir, E.; Makino, T.; Moraes, R.; Khan, I.; Pasco, D.S. Melanin: Dietary mucosal immune modulator from Echinacea and other botanical supplements. Int. Immunopharmacol. 2005, 5, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Timares, L. MC1R, eumelanin and pheomelanin: Their role in determining the susceptibility to skin cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swope, V.B.; Abdel-Malek, Z.A. MC1R: Front and center in the bright side of dark eumelanin and DNA repair. Int. J. Mol. Sci. 2018, 19, 2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premi, S. Role of Melanin Chemiexcitation in Melanoma Progression and Drug Resistance. Front. Oncol. 2020, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Tam, I.; Dzierżęga-Lęcznar, A.; Stępień, K. Differential expression of inflammatory cytokines and chemokines in lipopolysaccharide-stimulated melanocytes from lightly and darkly pigmented skin. Exp. Dermatol. 2019, 28, 551–560. [Google Scholar] [CrossRef]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilieș, M.; Hegheș, S.C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [Green Version]
- Sava, V.M.; Yang, S.M.; Hong, M.Y.; Yang, P.C.; Huang, G.S. Isolation and characterization of melanic pigments derived from tea and tea polyphenols. Food Chem. 2001, 73, 177–184. [Google Scholar] [CrossRef]
- Gerdemann, C.; Eicken, C.; Krebs, B. The crystal structure of catechol oxidase: New insight into the function of type-3 copper proteins. Acc. Chem. Res. 2002, 35, 183–191. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Mursalimov, S.R.; Gracheva, N.V.; Glagoleva, A.Y.; Börner, A.; Khlestkina, E.K. Melanin formation in barley grain occurs within plastids of pericarp and husk cells. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin pigment in plants: Current knowledge and future perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef]
- Dzierżęga-Lęcznar, A.; Kurkiewicz, S.; Stępień, K. Detection and quantitation of a pheomelanin component in melanin pigments using pyrolysis–gas chromatography/tandem mass spectrometry system with multiple reaction monitoring mode. J. Mass Spectrom. 2012, 47, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Chen, X.; Li, G.W.; Guo, G.Y.; Yang, L. Structural characteristics of pheomelanin-like pigment from Lachnum singerianum. In Advanced Materials Research; Trans Tech Publications Ltd.: Zurich, Switzerland, 2011; Volume 284, pp. 1742–1745. [Google Scholar]
- Pukalski, J.; Marcol, N.; Wolan, N.; Płonka, P.M.; Ryszka, P.; Kowalski, T.; Latowski, D. Detection of a pheomelanin-like pigment by EPR spectroscopy in the mycelium of Plenodomus biglobosus. Acta Biochim. Pol. 2020, 67, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Schmaler-Ripcke, J.; Sugareva, V.; Gebhardt, P.; Winkler, R.; Kniemeyer, O.; Heinekamp, T.; Brakhage, A.A. Production of pyomelanin, a second type of melanin, via the tyrosine degradation pathway in Aspergillus fumigatus. Appl. Environ. Microbiol. 2009, 75, 493–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolnicka-Glubisz, A.; Pecio, A.; Podkowa, D.; Kolodziejczyk, L.M.; Plonka, P.M. Pheomelanin in the skin of Hymenochirus boettgeri (Amphibia: Anura: Pipidae). Exp. Dermatol. 2012, 21, 535–561. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Zippin, J.H.; Ito, S. Chemical and biochemical control of skin pigmentation with special emphasis on mixed melanogenesis. Pigment Cell Melanoma Res. 2021, 34, 730–747. [Google Scholar] [CrossRef]
- Dzierżęga-Lęcznar, A.; Kurkiewicz, S.; Tam, I.; Marek, Ł.; Stępień, K. Pheomelanin content of cultured human melanocytes from lightly and darkly pigmented skin: A pyrolysis-gas chromatography/tandem mass spectrometry study. J. Anal. Appl. Pyrolysis 2017, 124, 349–354. [Google Scholar] [CrossRef]



| RT [min] | Compound | Group | RI RTX-5MS | RI NIST | CAS | Ech-mel % | Tyr-mel % |
|---|---|---|---|---|---|---|---|
| 6.97 | Benzene | B | 661 | 661 | 71-43-2 | 1.65 | 1.91 |
| 10.76 | Toluene | B | 767 | 767 | 108-88-3 | 5.10 | 6.68 |
| 14.52 | Ethylbenzene | B | 865 | 866 | 100-41-4 | 0.65 | 0.51 |
| 14.82 | m/p-Xylenes | B | 873 | 873 | 108-38-3/106-42-3 | 0.61 | 1.48 |
| 15.67 | Styrene | B | 895 | 895 | 100-42-5 | 2.00 | 1.90 |
| 15.75 | o-Xylene | B | 897 | 897 | 95-47-6 | 0.10 | 0.19 |
| 23.63 | Benzene, 1,2,3,4-tetramethyl- | B | 1166 | 1169 | 488-23-3 | 0.50 | - |
| B | 10.61 | 12.67 | |||||
| 23.33 | Benzyl nitrile | BN | 1151 | 1150 | 140-29-4 | 0.67 | 6.17 |
| 25.44 | Benzenepropanenitrile | BN | 1257 | 1250 | 645-59-0 | 0.16 | 0.46 |
| BN | 0.84 | 6.64 | |||||
| 10.41 | Pyrrole | Pyrr | 758 | 758 | 109-97-7 | 0.80 | 13.27 |
| 13.7 | 1H-Pyrrole, 2-methyl- | Pyrr | 844 | 853 | 636-41-9 | 0.83 | 5.25 |
| 14.06 | 1H-Pyrrole, 3-methyl- | Pyrr | 853 | 856 | 616-43-3 | 1.05 | 5.29 |
| 17.42 | 1H-Pyrrole, 2,5-dimethyl- | Pyrr | 942 | 937 | 625-84-3 | 0.21 | 0.22 |
| 17.73 | 1H-Pyrrole, 3-ethyl- | Pyrr | 951 | 950 | 1551-16-2 | 0.22 | 0.12 |
| 20.07 | 1H-Pyrrole, 2,3,5-trimethyl- | Pyrr | 1019 | - | 2199-41-9 | 0.14 | 0.04 |
| 20.82 | 1H-Pyrrole, 2-ethyl-4-methyl- | Pyrr | 1046 | - | 69687-77-0 | 0.28 | 0.03 |
| 22.27 | 1H-Pyrrole, 3-ethyl-2,4-dimethyl- | Pyrr | 1099 | - | 517-22-6 | 0.07 | - |
| 24.33 | 1H-Pyrrole, 3-ethyl-2,4,5-trimethyl- | Pyrr | 1200 | - | 520-69-4 | 0.31 | - |
| Pyrr | 3.90 | 24.23 | |||||
| 10.05 | Pyridine | Pr | 748 | 748 | 110-86-1 | 0.86 | 6.31 |
| 12.91 | Pyridine, 2-methyl- | Pr | 823 | 821 | 109-06-8 | 0.24 | 0.23 |
| Pr | 1.09 | 6.53 | |||||
| 18.98 | Fenol | Phe | 984 | 984 | 108-95-2 | 3.52 | 39.03 |
| 21.16 | o-Cresol | Phe | 1059 | 1058 | 95-48-7 | 0.25 | 0.15 |
| 21.66 | m/p-Cresol | Phe | 1077 | 1077 | 108-39-4/106-44-5 | 5.43 | 5.19 |
| 23.81 | Phenol, 4-ethyl- | Phe | 1175 | 1173 | 123-07-9 | 3.13 | 0.20 |
| 24.86 | 4-Winylofenol | Phe | 1227 | 1229 | 2628-17-3 | 3.16 | 0.57 |
| 29.65 | 2-Tert-Butyl-4-isopropyl-5-methylphenol | Phe | 1518 | - | - | 6.33 | - |
| Phe | 21.82 | 45.14 | |||||
| 22.12 | Phenol, 2-methoxy- | PPhe | 1094 | 1094 | 90-05-1 | 6.08 | 0.12 |
| 24.44 | Phenol, 2-methoxy-4-methyl- | PPhe | 1206 | 1207 | 93-51-6 | 3.07 | - |
| 24.52 | Catechol | PPhe | 1210 | 1210 | 120-80-9 | 22.03 | 1.77 |
| 26.1 | Phenol, 4-ethyl-2-methoxy- | PPhe | 1291 | 1260 | 2785-89-9 | 3.09 | - |
| 26.26 | 1,2-Benzenediol, 4-methyl- | PPhe | 1299 | 1295 | 452-86-8 | 4.32 | - |
| 26.74 | 2-Methoxy-4-vinylphenol | PPhe | 1330 | 1330 | 7786-61-0 | 10.49 | - |
| 27.33 | Phenol, 2,6-dimethoxy- | PPhe | 1367 | 1367 | 91-10-1 | 1.61 | - |
| 27.44 | Phenol, 2-methoxy-4-(2-propenyl)- | PPhe | 1374 | 1374 | 97-53-0 | 0.14 | - |
| 27.82 | 4-Ethylcatechol | PPhe | 1397 | 1392 | 1124-39-6 | 8.10 | - |
| 32.28 | Phenol, 2,6-dimethoxy-4-(1E)-1-propen-1-yl- | PPhe | 1720 | 1704 | 20675-95-0 | 0.84 | - |
| PPhe | 59.76 | 1.89 | |||||
| 26.49 | 1H-Indole | Ind | 1314 | 1316 | 120-72-9 | 1.10 | 2.08 |
| 28.07 | Methylindole | Ind | 1414 | - | - | 0.33 | 0.57 |
| Ind | 1.43 | 2.65 | |||||
| 8.48 | Furan, 2,5-dimethyl- | Fur | 707 | 708 | 625-86-5 | 0.39 | 0.26 |
| 16.47 | 1-(2-Furanylo)-etanon | Fur | 917 | 917 | 1192-62-7 | 0.15 | - |
| Fur | 0.54 | 0.26 |
| No. | RT [min] | Compound | RI RTX-5MS | MS Transition * |
|---|---|---|---|---|
| 1 | 9.6 | Thiazole | 736.7 | 85 -> 58 |
| 2 | 25.3 | Benzothiazole | 1250.0 | 135 -> 108 |
| 3 | 27.7 | 4-Hydroxybenzothiazole | 1391.8 | 151 -> 96 |
| 4 | 28.9 | 2,3-Dihydro-5H-1,4-benzothiazin-5-one (isomer 1) | 1470.8 | 165 -> 136 |
| 5 | 29.3 | 2,3-Dihydro-5H-1,4-benzothiazin-5-one (isomer 2) | 1490.3 | 165 -> 136 |
| 6 | 29.4 | 2,3-Dihydro-5H-1,4-benzothiazin-5-one (isomer 3) | 1501.5 | 165 -> 136 |
| 7 | 30.3 | 7-Methy-2,3-dihydro-5H-1,4-benzothiazi-5-one (isomer 1) | 1566.7 | 178 -> 109 |
| 8 | 30.5 | 7-Methy-2,3-dihydro-5H-1,4-benzothiazi-5-one (isomer 2) | 1581.2 | 178 -> 109 |
| 9 | 30.6 | 4-Hydroxy-6-ethylbenzothiazole (isomer 1) | 1584.8 | 179 -> 164 |
| 10 | 30.9 | 4-Hydroxy-6-ethylbenzothiazole (isomer 2) | 1605.5 | 179 -> 164 |
| 11 | 31.4 | 7-Methyl-5H-1,4-benzothiazin-5-one | 1647.3 | 177 -> 121 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurkiewicz, S.; Marek, Ł.; Kurkiewicz, M.; Kurkiewicz, A.; Dzierżęga-Lęcznar, A. Are Plants Capable of Pheomelanin Synthesis? Gas Chromatography/Tandem Mass Spectrometry Characterization of Thermally Degraded Melanin Isolated from Echinacea purpurea. Processes 2022, 10, 2465. https://doi.org/10.3390/pr10112465
Kurkiewicz S, Marek Ł, Kurkiewicz M, Kurkiewicz A, Dzierżęga-Lęcznar A. Are Plants Capable of Pheomelanin Synthesis? Gas Chromatography/Tandem Mass Spectrometry Characterization of Thermally Degraded Melanin Isolated from Echinacea purpurea. Processes. 2022; 10(11):2465. https://doi.org/10.3390/pr10112465
Chicago/Turabian StyleKurkiewicz, Slawomir, Łukasz Marek, Małgorzata Kurkiewicz, Adam Kurkiewicz, and Anna Dzierżęga-Lęcznar. 2022. "Are Plants Capable of Pheomelanin Synthesis? Gas Chromatography/Tandem Mass Spectrometry Characterization of Thermally Degraded Melanin Isolated from Echinacea purpurea" Processes 10, no. 11: 2465. https://doi.org/10.3390/pr10112465
APA StyleKurkiewicz, S., Marek, Ł., Kurkiewicz, M., Kurkiewicz, A., & Dzierżęga-Lęcznar, A. (2022). Are Plants Capable of Pheomelanin Synthesis? Gas Chromatography/Tandem Mass Spectrometry Characterization of Thermally Degraded Melanin Isolated from Echinacea purpurea. Processes, 10(11), 2465. https://doi.org/10.3390/pr10112465