Thermal Safety Evaluation of Silane Polymer Compounds as Electrolyte Additives for Silicon-Based Anode Lithium-Ion Batteries
Abstract
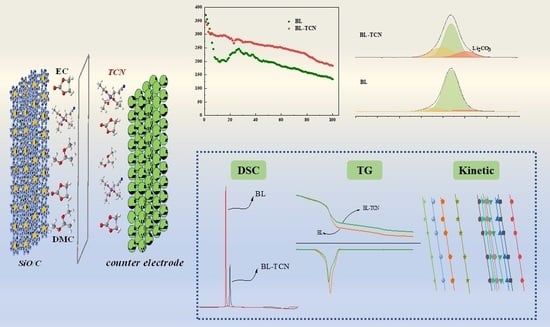
:1. Introduction
2. Materials and Methods
2.1. Preparation of Electrolytes and Electrodes
2.2. Electrochemical Measurements
2.3. XPS Measurement
2.4. Differential Scanning Calorimetry and Thermogravimetric Analysis
2.5. Thermokinetic Analysis
2.5.1. KAS Method
2.5.2. FWO Method
2.5.3. Starink Method
3. Results and Discussion
3.1. Electrochemical Performance and Interface Properties
3.2. Analysis of DSC
3.3. Thermogravimetric Analysis
3.4. Thermokinetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yuan, M.; Liu, K. Rational design on separators and liquid electrolytes for safer lithium-ion batteries. J. Energy Chem. 2020, 43, 58–70. [Google Scholar] [CrossRef]
- Wu, Z.H.; Huang, A.C.; Tang, Y.; Yang, Y.P.; Liu, Y.C.; Li, Z.P.; Zhou, H.L.; Huang, C.F.; Xing, Z.X.; Shu, C.M.; et al. Thermal Effect and Mechanism Analysis of Flame-Retardant Modified Polymer Electrolyte for Lithium-Ion Battery. Polymers 2021, 13, 1675. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Sun, S.; He, D.; Li, P.; Liu, Y.; Wan, Q.; Tan, Q.; Liu, Z.; An, F.; Gong, G.; Qu, X. Improved adhesion of cross-linked binder and SiO2-coating enhances structural and cyclic stability of silicon electrodes for lithium-ion batteries. J. Power Sources 2020, 454, 227907. [Google Scholar] [CrossRef]
- Wang, D.; Gao, M.; Pan, H.; Liu, Y.; Wang, J.; Li, S.; Ge, H. Enhanced cycle stability of micro-sized Si/C anode material with low carbon content fabricated via spray drying and in situ carbonization. J. Alloy. Compd. 2014, 604, 130–136. [Google Scholar] [CrossRef]
- Shen, J.; Chen, H.; Yu, L.; Huang, D.; Luo, Z. 4, 5-difluoro-1, 3-dioxolan-2-one as an film forming additive on LiNi0.8Co0.15Al0.05O2/SiO@C full cells. J. Electroanal. Chem. 2019, 834, 1–7. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Jiang, J.-C.; Huang, A.-C.; Tang, Y.; Xie, L.-J.; Zhai, J.; Xing, Z.-X. A novel multifunctional additive strategy improves the cycling stability and thermal stability of SiO/C anode Li-ion batteries. Process. Saf. Environ. Prot. 2022, 164, 555–565. [Google Scholar] [CrossRef]
- Nigl, T.; Baldauf, M.; Hohenberger, M.; Pomberger, R. Lithium-Ion Batteries as Ignition Sources in Waste Treatment Processes—A Semi-Quantitate Risk Analysis and Assessment of Battery-Caused Waste Fires. Processes 2020, 9, 49. [Google Scholar] [CrossRef]
- Profatilova, I.A.; Stock, C.; Schmitz, A.; Passerini, S.; Winter, M. Enhanced thermal stability of a lithiated nano-silicon electrode by fluoroethylene carbonate and vinylene carbonate. J. Power Sources. 2013, 222, 140–149. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Jiang, J.-C.; Huang, A.-C.; Tang, Y.; Liu, Y.-C.; Xie, L.-J.; Zhang, C.-Z.; Wu, Z.-h.; Xing, Z.-X.; Yu, F. 3-(Trifluoromethyl)benzoylacetonitrile: A multi-functional safe electrolyte additive for LiNi0.8Co0.1Mn0.1O2 cathode of high voltage lithium-ion battery. Process. Saf. Environ. Prot. 2022, 160, 80–90. [Google Scholar] [CrossRef]
- Profatilova, I.A.; Kim, S.-S.; Choi, N.-S. Enhanced thermal properties of the solid electrolyte interphase formed on graphite in an electrolyte with fluoroethylene carbonate. Electrochim. Acta. 2009, 54, 4445–4450. [Google Scholar] [CrossRef]
- Zhang, S.S. Electrochemical study of the formation of a solid electrolyte interface on graphite in a LiBC2O4F2-based electrolyte. J. Power Sources 2007, 163, 713–718. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Zhang, H. Effects of electrolyte additive on the electrochemical performance of Si/C anode for lithium-ion batteries. Ionics 2018, 24, 3691–3698. [Google Scholar] [CrossRef]
- Xu, N.; Sun, Y.; Shi, J.; Chen, J.; Liu, G.; Zhou, K.; He, H.; Zhu, J.; Zhang, Z.; Yang, Y. Fluorinated cyclic siloxane additives for high energy density Li-ion batteries with high nickel cathodes and silicon–carbon anodes. J. Power Sources 2021, 511, 230437. [Google Scholar] [CrossRef]
- Schiele, A.; Breitung, B.; Hatsukade, T.; Berkes, B.B.; Hartmann, P.; Janek, J.; Brezesinski, T. The Critical Role of Fluoroethylene Carbonate in the Gassing of Silicon Anodes for Lithium-Ion Batteries. ACS Energy Lett. 2017, 2, 2228–2233. [Google Scholar] [CrossRef]
- Aupperle, F.; Eshetu, G.G.; Eberman, K.W.; Xioa, A.; Bridel, J.-S.; Figgemeier, E. Realizing a high-performance LiNi0.6Mn0.2Co0.2O2/silicon–graphite full lithium ion battery cell via a designer electrolyte additive. J. Mater. Chem. A 2020, 8, 19573–19587. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Z.; Xu, Y.; Li, Z.; Yao, N.; Wang, J.; Feng, Z.; Wang, K.; Xie, J.; Zhao, H. Synergistic effect of vinylene carbonate (VC) and LiNO3 as functional additives on interphase modulation for high performance SiO anodes. J. Power Sources 2021, 514, 230595. [Google Scholar] [CrossRef]
- Xie, L.-J.; Jiang, J.-C.; Huang, A.-C.; Tang, Y.; Liu, Y.-C.; Zhou, H.-L.; Xing, Z.-X. Calorimetric Evaluation of Thermal Stability of Organic Liquid Hydrogen Storage Materials and Metal Oxide Additives. Energies 2022, 15, 2236. [Google Scholar] [CrossRef]
- Huang, A.-C.; Li, Z.-P.; Liu, Y.-C.; Tang, Y.; Huang, C.-F.; Shu, C.-M.; Xing, Z.-X.; Jiang, J.-C. Essential hazard and process safety assessment of para-toluene sulfonic acid through calorimetry and advanced thermokinetics. J. Loss Prev. Process. Ind. 2021, 72, 104558. [Google Scholar] [CrossRef]
- Taco-Vasquez, S.; Ron, C.A.; Murillo, H.A.; Chico, A.; Arauz, P.G. Thermochemical Analysis of a Packed-Bed Reactor Using Finite Elements with FlexPDE and COMSOL Multiphysics. Processes 2022, 10, 1144. [Google Scholar] [CrossRef]
- Huang, A.-C.; Liao, F.-C.; Huang, C.-F.; Tang, Y.; Zhang, Y.; Shu, C.-M.; Xing, Z.-X.; Jiang, J.-C.; Hsieh, W.-Y. Calorimetric approach to establishing thermokinetics for cosmeceutical benzoyl peroxides containing metal ions. J. Therm. Anal. Calorim. 2021, 144, 373–382. [Google Scholar] [CrossRef]
- Li, Z.P.; Jiang, J.C.; Huang, A.C.; Tang, Y.; Miao, C.F.; Zhai, J.; Huang, C.F.; Xing, Z.X.; Shu, C.M. Thermal hazard evaluation on spontaneous combustion characteristics of nitrocellulose solution under different atmospheric conditions. Sci. Rep. 2021, 11, 24053. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.B.; Almeida, J.S.; Barbosa, R.V.; Fernandes, G.J.T.; Coriolano, A.C.F.; Fernandes, V.J.; Araujo, A.S. Thermal Oxidative Stability of Biodiesel/Petrodiesel Blends by Pressurized Differential Scanning Calorimetry and Its Calculated Cetane Index. Processes 2021, 9, 174. [Google Scholar] [CrossRef]
- Merighi, S.; Mazzocchetti, L.; Benelli, T.; Giorgini, L. Evaluation of Novel Bio-Based Amino Curing Agent Systems for Epoxy Resins: Effect of Tryptophan and Guanine. Processes 2020, 9, 42. [Google Scholar] [CrossRef]
- Yao, C.; Liu, Y.-C.; Wu, J.; Tang, Y.; Zhai, J.; Shu, C.-M.; Jiang, J.-C.; Xing, Z.-X.; Huang, C.-F.; Huang, A.-C. Thermal Stability Determination of Propylene Glycol Sodium Alginate and Ammonium Sulfate with Calorimetry Technology. Processes 2022, 10, 1177. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Dong, X.-L.; Tang, Y.; Huang, A.-C.; Chen, F.; Kang, Q.-C.; Shu, Z.-J.; Xing, Z.-X. Experimental investigations of extinguishing sodium pool fires using modified expandable graphite powders. Case Stud. Therm. Eng. 2022, 32, 101911. [Google Scholar] [CrossRef]
- Yin, X.-Y.; Liu, T.; Liu, Y.-C.; Tang, Y.; Huang, A.-C.; Dong, X.-L.; Liu, Y.-J. Feasibility Study of Fine Water Mist Applied to Cold Storage Fire Protection. Processes 2022, 10, 1533. [Google Scholar] [CrossRef]
- Chen, F.; Dong, X.-L.; Tang, Y.; Huang, A.-C.; Zhang, M.-L.; Kang, Q.-C.; Shu, Z.-J.; Xing, Z.-X. Thermal Characteristic Analysis of Sodium in Diluted Oxygen via Thermogravimetric Approach. Processes 2022, 10, 704. [Google Scholar] [CrossRef]
- Huang, A.-C.; Huang, C.-F.; Tang, Y.; Xing, Z.-X.; Jiang, J.-C. Evaluation of multiple reactions in dilute benzoyl peroxide concentrations with additives using calorimetric technology. J. Loss Prev. Process. Ind. 2021, 69, 104373. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional Kinetic Analysis of Thermally Stimulated Processes in Polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Yang, N.; Jiang, J.-C.; Huang, A.-C.; Tang, Y.; Li, Z.-P.; Cui, J.-W.; Shu, C.-M.; Xing, Z.-X. Thermokinetic model-based experimental and numerical investigation of the thermal hazards of nitrification waste. J. Loss Prev. Process. Ind. 2022, 79, 104836. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Huang, A.-C.; Tang, Y.; Huang, C.-F.; Shen, Q.; Shu, C.-M.; Xing, Z.-X.; Jiang, J.-C. Thermokinetic analysis of the stability of acetic anhydride hydrolysis in isothermal calorimetry techniques. J. Therm. Anal. Calorim. 2021, 147, 7865–7873. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Huang, A.-C.; Tang, Y.; Ma, X.-M.; Yang, Y.-P.; Wu, Z.-H.; Shu, C.-M.; Xing, Z.-X.; Jiang, J.-C. Thermokinetic model establishment and numerical simulation of 2,4,6-trinitrophenol based on eco-friendly synthesis method. J. Energetic Mater. 2021, 1–20. [Google Scholar] [CrossRef]
- Yepes, C.; Estévez, J.; Arroyo, M.; Ladero, M. Immobilization of an Industrial β-Glucosidase from Aspergillus fumigatus and Its Use for Cellobiose Hydrolysis. Processes 2022, 10, 1225. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta. 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Chang, T.; Hsueh, K.-H.; Liu, C.-C.; Cao, C.-R.; Shu, C.-M. A Method to Derive the Characteristic and Kinetic Parameters of 1,1-Bis(tert-butylperoxy)cyclohexane from DSC Measurements. Processes 2022, 10, 1026. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Jiang, J.-C.; Huang, A.-C.; Tang, Y.; Yang, Y.-P.; Zhou, H.-L.; Zhai, J.; Xing, Z.-X.; Huang, C.-F.; Shu, C.-M. Hazard assessment of the thermal stability of nitrification by-products by using an advanced kinetic model. Process. Saf. Environ. Prot. 2022, 160, 91–101. [Google Scholar] [CrossRef]









| Sample | To (°C) | Tp (°C) | ΔH (J/g) |
|---|---|---|---|
| Lithiated anode with BL | 122.22 | 132.25 | 240.48 |
| Lithiated anode with BL-TCN | 127.07 | 133.52 | 151.71 |
| α | Ea (kJ/mol) | R2 | ||||
|---|---|---|---|---|---|---|
| KAS | FWO | Starink | KAS | FWO | Starink | |
| 0.05 | 91.93 | 91.56 | 91.69 | 0.9595 | 0.9654 | 0.9698 |
| 0.10 | 87.59 | 92.36 | 91.65 | 0.9485 | 0.9572 | 0.9602 |
| 0.20 | 89.30 | 91.37 | 90.92 | 0.9664 | 0.9368 | 0.9430 |
| 0.30 | 90.70 | 89.95 | 89.22 | 0.9084 | 0.9070 | 0.9004 |
| 0.40 | 90.69 | 92.12 | 90.96 | 0.9392 | 0.9683 | 0.9400 |
| 0.50 | 94.59 | 93.30 | 93.52 | 0.9637 | 0.9518 | 0.9643 |
| 0.60 | 89.47 | 91.18 | 89.84 | 0.9445 | 0.9621 | 0.9453 |
| 0.70 | 86.69 | 92.15 | 91.45 | 0.9561 | 0.9735 | 0.9568 |
| 0.80 | 89.98 | 91.60 | 91.05 | 0.9422 | 0.9496 | 0.9428 |
| 0.90 | 92.65 | 93.71 | 89.59 | 0.9694 | 0.9542 | 0.9832 |
| 0.95 | 94.52 | 92.18 | 90.89 | 0.9165 | 0.9238 | 0.9186 |
| 0.99 | 92.42 | 94.29 | 90.45 | 0.9694 | 0.9128 | 0.9832 |
| Average | 90.88 | 92.15 | 90.94 | 0.9487 | 0.9469 | 0.9506 |
| Lithiated Anode with BL | Lithiated Anode with BL-TCN | |||
|---|---|---|---|---|
| Ea (kJ/mol) | R2 | Ea (kJ/mol) | R2 | |
| KAS | 68.47 | 0.9558 | 90.88 | 0.9487 |
| FWO | 69.51 | 0.9637 | 92.15 | 0.9469 |
| Starink | 67.39 | 0.9426 | 90.94 | 0.9506 |
| Average | 68.46 | 0.9540 | 91.32 | 0.9487 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.-Z.; Xie, L.-J.; Tang, Y.; Li, Y.; Jiang, J.-C.; Huang, A.-C. Thermal Safety Evaluation of Silane Polymer Compounds as Electrolyte Additives for Silicon-Based Anode Lithium-Ion Batteries. Processes 2022, 10, 1581. https://doi.org/10.3390/pr10081581
Zhang C-Z, Xie L-J, Tang Y, Li Y, Jiang J-C, Huang A-C. Thermal Safety Evaluation of Silane Polymer Compounds as Electrolyte Additives for Silicon-Based Anode Lithium-Ion Batteries. Processes. 2022; 10(8):1581. https://doi.org/10.3390/pr10081581
Chicago/Turabian StyleZhang, Chuan-Zhu, Lin-Jie Xie, Yan Tang, You Li, Jun-Cheng Jiang, and An-Chi Huang. 2022. "Thermal Safety Evaluation of Silane Polymer Compounds as Electrolyte Additives for Silicon-Based Anode Lithium-Ion Batteries" Processes 10, no. 8: 1581. https://doi.org/10.3390/pr10081581
APA StyleZhang, C. -Z., Xie, L. -J., Tang, Y., Li, Y., Jiang, J. -C., & Huang, A. -C. (2022). Thermal Safety Evaluation of Silane Polymer Compounds as Electrolyte Additives for Silicon-Based Anode Lithium-Ion Batteries. Processes, 10(8), 1581. https://doi.org/10.3390/pr10081581