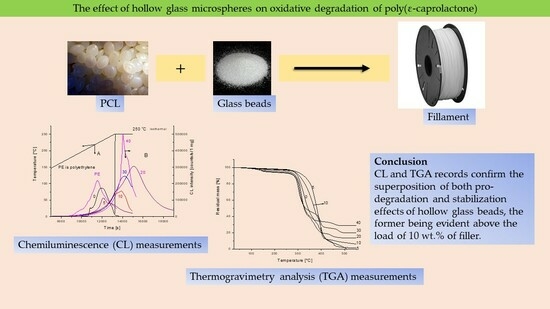
The Effect of Hollow Glass Microspheres on the Kinetics of Oxidation of Poly(ε-Caprolactone) Determined from Non-Isothermal Thermogravimetry and Chemiluminescence
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. PCL Filament Preparation
2.3. Thermogravimetry
2.4. Chemiluminescence
3. Results and Discussion
3.1. Chemiluminescence and Thermogravimetry Characterization of Hollow Glass Microspheres
3.2. Oxidative Degradation of PCL in the Presence of Hollow Glass Beads
3.2.1. Chemiluminescence
3.2.2. Thermogravimetry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Soto, M.; Gordillo, A.; Maspoch, M.L.L.; Velasco, J.I.; Santana, O.O.; Martí, A.B. Glass bead filled polystyrene composites: Morphology and fracture. Polym. Bull. 2002, 47, 587–594. [Google Scholar] [CrossRef]
- Ashby, G.E. Oxyluminescence from polypropylene. J. Polym. Sci. 1961, 50, 99–106. [Google Scholar] [CrossRef]
- Schard, M.P.; Russell, C.A. Oxyluminescence of polymers. II. Effect of temperature and antioxidants. J. Appl. Polym. Sci. 1964, 8, 997–1006. [Google Scholar] [CrossRef]
- Matisová-Rychlá, L.; Rychlý, J.; George, G.A. Chemiluminescence from thermal oxidation of poly (2,6-dimethyl-1,4-phenylene oxide). Polym. Degrad. Stab. 2002, 75, 385–396. [Google Scholar] [CrossRef]
- Billingham, N.C.; Then, E.T.H.; Gijsman, P.J. Chemiluminescence from peroxides in polypropylene. Part I: Relation of luminescence to peroxide content. Polym. Degrad. Stab. 1991, 34, 263–277. [Google Scholar] [CrossRef]
- George, G.A.; Egglestone, G.T.; Riddell, S.Z. Chemiluminescence studies of the degradation and stabilization of polymers. Polym. Eng. Sci. 1983, 23, 412–418. [Google Scholar] [CrossRef]
- Kometani, H.; Matsumura, T.; Suga, T.; Kanai, T. Quantitative analysis for polymer degradation in the extrusion process. Int. Polym. Process. 2006, 21, 24–31. [Google Scholar] [CrossRef]
- Sivalingam, G.; Madras, G. Thermal degradation of poly (ε-caprolactone). Polym. Degrad. Stab. 2003, 80, 11–16. [Google Scholar] [CrossRef]
- Vignali, A.; Iannace, S.; Falcone, G.; Utzeri, R.; Stagnaro, P.; Bertini, F. Lightweight poly(ε-caprolactone) composites with surface modified hollow glass microspheres for use in rotational molding: Thermal, Rheological and Mechanical Properties. Polymers 2019, 11, 624. [Google Scholar] [CrossRef]
- Kovácová, M.; Vykydalová, A.; Spitálsky, Z. Polycaprolactone with glass beads for 3D printing filaments. Processes 2023, 11, 395. [Google Scholar] [CrossRef]
- Moghadam, S.G.; Pazokifard, S.; Mirabedini, S. Silane treatment of drop-on glass-beads and their performance in two-component traffic paints. Prog. Org. Coat. 2021, 156, 106235. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchell, J.W. Effects of inorganic fillers on the thermal and mechanical properties of poly(lactic acid), Hindawi Publishing Corporation. Int. J. Polym. Sci. 2014, 2014, 827028. [Google Scholar] [CrossRef]
- Zlatkevich, L.; Burlett, D.J. Chemiluminescence in studying the thermal oxidation of rubber compounds. Polym. Degrad. Stab. 1999, 65, 53–58. [Google Scholar] [CrossRef]
- Tarrío-Saavedra, J.; López-Beceiro, J.; Naya, S.; Artiaga, R. Effect of silica content on thermal stability of fumed silica/epoxy composites. Polym. Degrad. Stab. 2008, 93, 2133–2137. [Google Scholar] [CrossRef]
- Nikkhah, S.J.; Ramazani, S.A.; Baniasadi, H.; Tavakolzadeh, F. Investigation of properties of polyethylene/clay nanocomposites prepared by new in situ Ziegler–Natta catalyst. Mater. Des. 2009, 30, 2309–2315. [Google Scholar] [CrossRef]
- Suraci, S.V.; Fabiani, D.; Roland, S.; Colin, X. Multi scale aging assessment of low-voltage cables subjected to radio-chemical aging: Towards an electrical diagnostic technique. Polym. Test. 2021, 103, 107352. [Google Scholar] [CrossRef]
- Zuo, J.; Yao, Z.; Zhou, J. Mechanical and thermal properties of phenolic foams reinforced by hollow glass beads. Adv. Mater. Res. 2014, 988, 13–22. [Google Scholar] [CrossRef]
- Rychlý, J.; Matisová-Rychlá, L.; Csomorová, K.; Janigová, I.; Schilling, M.; Learner, T. Non˗isothermal thermogravimetry, differential scanning calorimetry and chemiluminescence in degradation of polyethylene, polypropylene, polystyrene and poly(methyl metacrylate). Polym. Degrad. Stab. 2011, 96, 1573–1581. [Google Scholar] [CrossRef]
- Agrawal, R.K. The compensation effect: A fact or a fiction. J. Therm. Anal. 1989, 35, 909–917. [Google Scholar] [CrossRef]
- Agrawal, R.K. The compensation effect. J. Therm. Anal. Calorim. 1988, 34, 1141–1149. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.-X. Isokinetic relationship, isoequilibrium relationship, and Enthalpy˗Entropy compensation. Chem. Rev. 2001, 101, 673–696. [Google Scholar] [CrossRef] [PubMed]













| The Rate of Heating, °C/min | The Amount of Additive, wt.% | Temperature of 85% Residual Mass, °C | Temperature of 70% Residual Mass, °C | Temperature of 50% Residual Mass, °C |
|---|---|---|---|---|
| 2.5 | 0 | 292 | 273 | 262 |
| 5 | 360 | 342 | 318 | |
| 10 | 323 | 302 | 281 | |
| 20 | 285 | 277 | 268 | |
| 30 | 289 | 275 | 266 | |
| 40 | 295 | 273 | 285 | |
| 5 | 0 | 312 | 298 | 288 |
| 5 | 373 | 360 | 337 | |
| 10 | 345 | 323 | 301 | |
| 20 | 302 | 295 | 288 | |
| 30 | 303 | 290 | 282 | |
| 40 | 307 | 290 | 283 | |
| 10 | 0 | 347 | 324 | 309 |
| 5 | 382 | 366 | 345 | |
| 10 | 371 | 349 | 323 | |
| 20 | 329 | 314 | 303 | |
| 30 | 345 | 309 | 299 | |
| 40 | 332 | 305 | 295 |
| Sample The Rate of Heating, °C/min Rate Constant at 250 °C, s−1 | α1 | A1/β, °C−1 | E1, J/mol | α2 | A2/β, °C−1 | E2, J/mol | α3 | A3/β, °C−1 | E3, J/mol |
|---|---|---|---|---|---|---|---|---|---|
| PCL | |||||||||
| 2.5 | 0.421 | 4.6 × 1018 | 207,145 | 0.402 | 1.68 × 107 | 100,165 | 0.179 | 114.2 | 55,658 |
| k250 | 5.4 × 10−4 | 8.1 × 10−5 | 1.4 × 10−5 | ||||||
| 5 | 0.461 | 1.64 × 1019 | 223,437 | 0.399 | 4.8 × 107 | 108,666 | 0.141 | 395.0 | 65,421 |
| k250 | 9.4 × 10−5 | 6.7 × 10−5 | 1.1 × 10−5 | ||||||
| 10 | 0.456 | 1.13 × 1013 | 164,128 | 0.392 | 7.4 × 107 | 118,527 | 0.152 | 1.74 × 106 | 116,665 |
| k250 | 9.9 × 10−5 | 2.1 × 10−5 | 7.8 × 10−7 | ||||||
| PC L 5 wt.% | |||||||||
| 2.5 | 0.077 | 1.36 × 1016 | 195,239 | 0.525 | 2.15 × 1016 | 214,020 | 0.397 | 59.99 | 47,330 |
| k250 | 2.4 × 10−5 | 5.3 × 10−7 | 5.0 × 10−5 | ||||||
| 5 | 0.043 | 1.36 × 1017 | 206,985 | 0.673 | 3.03 × 1017 | 195,915 | 0.286 | 4.79 | 37,616 |
| k250 | 3.3 × 10−5 | 4.6 × 10−4 | 3.7 × 10−5 | ||||||
| 10 | 0.011 | 5.2 × 105 | 84,937 | 0.767 | 1.49 × 1011 | 158,238 | 0.223 | 2.2 | 34,460 |
| k250 | 3.3 × 10−4 | 5.0 × 10−6 | 1.4 × 10−4 | ||||||
| PCL 10 wt.% | |||||||||
| 2.5 | 0.694 | 2.05 × 107 | 99,633 | 0.151 | 5.06 × 106 | 99,090 | 0.155 | 4.86 | 49,498 |
| k250 | 1.1 × 10−4 | 3.1 × 10−5 | 2.7 × 10−6 | ||||||
| 5 | 0.414 | 4.18 × 107 | 110,945 | 0.421 | 7.64 × 107 | 107,862 | 0.165 | 4.02 | 48,910 |
| k250 | 3.4 × 10−5 | 1.3 × 10−4 | 4.7 × 10−6 | ||||||
| 10 | 0.429 | 6.21 × 108 | 121,296 | 0.374 | 2.73 × 1019 | 262,937 | 0.196 | 0.31 | 28,423 |
| k250 | 9.6 × 10−5 | 3.8 × 10−8 | 7.8 × 10−5 | ||||||
| PCL 20 wt.% | |||||||||
| 2.5 | 0.731 | 2.9 × 1017 | 198,995 | 0.053 | 6140 | 70,620 | 0.219 | 4.33 × 10−4 | 2.03 |
| k250 | 2.2 × 10−4 | 2.5 × 10−5 | 1.8 × 10−5 | ||||||
| 5 | 0.54 | 8.6 × 1023 | 262,268 | 0.190 | 1.79 × 1011 | 143,724 | 0.272 | 2.49 × 10−2 | 18,349 |
| k250 | 6.9 × 10−4 | 8.3 × 10−4 | 3.1 × 10−5 | ||||||
| 10 | 0.448 | 2.0 × 1021 | 252,414 | 0.255 | 1.32 × 1010 | 141,856 | 0.296 | 2.1 × 10−2 | 14,094 |
| k250 | 3.1 × 10−5 | 1.9 × 10−5 | 1.4 × 10−4 | ||||||
| PCL 30 wt.% | |||||||||
| 2.5 | 0.563 | 3.5 × 1017 | 198,339 | 0.138 | 100.9 | 46,203 | 0.301 | 2.9 × 10−4 | 29.8 |
| k250 | 3.1 × 10−4 | 1.1 × 10−4 | 1.2 × 10−5 | ||||||
| 5 | 0.469 | 1.9 × 1022 | 253,646 | 0.121 | 7.9 × 107 | 109,662 | 0.408 | 1.4 × 10−2 | 12,910 |
| k250 | 1.1 × 10−4 | 8.7 × 10−5 | 6.1 × 10−5 | ||||||
| 10 | 0.399 | 2.65 × 1022 | 262,532 | 0.195 | 2.69 × 1015 | 212,838 | 0.407 | 1.25 × 10−2 | 11,529 |
| k250 | 4.0 × 10−5 | 3.5 × 10−7 | 1.5 × 10−4 | ||||||
| PCL 40 wt.% | |||||||||
| 2.5 | 0.553 | 1.48 × 1012 | 143,528 | 0.061 | 2.11 × 109 | 137,902 | 0.383 | 3.4 × 10−4 | 38.5 |
| k250 | 3.5 × 10−4 | 1.8 × 10−5 | 1.4 × 10−5 | ||||||
| 5 | 0.572 | 1.15 × 1015 | 178,447 | 0.065 | 5.88 × 107 | 118,565 | 0.369 | 4.4 × 10−4 | 9.04 |
| k250 | 1.8 × 10−4 | 8.5 × 10−6 | 3.6 × 10−5 | ||||||
| 10 | 0.315 | 4.15 × 1022 | 262,571 | 0.211 | 1.41 × 1012 | 157,765 | 0.477 | 2.7 × 10−3 | 5676 |
| k250 | 6.2 × 10−5 | 5.3 × 10−5 | 1.2 × 10−4 |
| Line | Straight Line | Isokinetic Temperature, °C |
|---|---|---|
| 1 | 28,048–4399/T | 252.5 |
| 2 | 22,914–5005/T | 325.0 |
| 3 | 5081/T–5081/T | 334.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vykydalová, A.; Špitálský, Z.; Kováčová, M.; Rychlý, J. The Effect of Hollow Glass Microspheres on the Kinetics of Oxidation of Poly(ε-Caprolactone) Determined from Non-Isothermal Thermogravimetry and Chemiluminescence. Processes 2023, 11, 3372. https://doi.org/10.3390/pr11123372
Vykydalová A, Špitálský Z, Kováčová M, Rychlý J. The Effect of Hollow Glass Microspheres on the Kinetics of Oxidation of Poly(ε-Caprolactone) Determined from Non-Isothermal Thermogravimetry and Chemiluminescence. Processes. 2023; 11(12):3372. https://doi.org/10.3390/pr11123372
Chicago/Turabian StyleVykydalová, Anna, Zdenko Špitálský, Mária Kováčová, and Jozef Rychlý. 2023. "The Effect of Hollow Glass Microspheres on the Kinetics of Oxidation of Poly(ε-Caprolactone) Determined from Non-Isothermal Thermogravimetry and Chemiluminescence" Processes 11, no. 12: 3372. https://doi.org/10.3390/pr11123372
APA StyleVykydalová, A., Špitálský, Z., Kováčová, M., & Rychlý, J. (2023). The Effect of Hollow Glass Microspheres on the Kinetics of Oxidation of Poly(ε-Caprolactone) Determined from Non-Isothermal Thermogravimetry and Chemiluminescence. Processes, 11(12), 3372. https://doi.org/10.3390/pr11123372