Anaerobic Co-Digestion of Sewage Sludge and Trade Wastes: Beneficial and Inhibitory Effects of Individual Constituents
Abstract
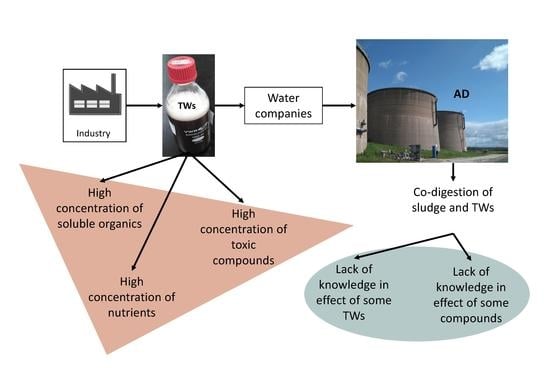
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum
2.2. Trade Wastes Data Analysis
2.3. Batch Tests
2.4. Analytical Methods
2.5. Trade Wastes Load Calculation
2.6. Statistics
3. Results and Discussion
3.1. Trade Wastes Characterisation
3.2. Biological Methane Potential Tests
3.2.1. Yield
Ammonia
Zinc
Copper
Aluminium
Nitrate
Arsenic and Mercury
Counter-Ion Effect
3.2.2. Kinetics
3.3. Potential for Co-Digestion of Trade Wastes
4. Conclusions
5. Disclaimer
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berenjkar, P.; Islam, M.; Yuan, Q. Co-treatment of sewage sludge and mature landfill leachate by anaerobic digestion. Int. J. Environ. Sci. Technol. 2019, 16, 2465–2474. [Google Scholar] [CrossRef]
- Cardona, L.; Levrard, C.; Guenne, A.; Chapleur, O.; Mazéas, L. Co-digestion of wastewater sludge: Choosing the optimal blend. Waste Manag. 2019, 87, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Maragkaki, A.E.; Fountoulakis, M.; Gypakis, A.; Kyriakou, A.; Lasaridi, K.; Manios, T. Pilot-scale anaerobic co-digestion of sewage sludge with agro-industrial by-products for increased biogas production of existing digesters at wastewater treatment plants. Waste Manag. 2017, 59, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering Treatment and Reuse, 4th ed.; Metcalf & Eddy, Inc.: McGraw Hill, NY, USA, 1991. [Google Scholar]
- Silvestre, G.; Rodríguez-Abalde, A.; Fernández, B.; Flotats, X.; Bonmatí, A. Biomass adaptation over anaerobic co-digestion of sewage sludge and trapped grease waste. Bioresour. Technol. 2011, 102, 6830–6836. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Ma, Y. New insights into the co-locating concept on synergistic co-digestion of sewage sludge and food waste towards energy self-sufficient in future WWTPs. Bioresour. Technol. Rep. 2020, 10, 100351. [Google Scholar] [CrossRef]
- Nielfa, A.; Cano, R.; Fdz-Polanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5 (Suppl. C), 14–21. [Google Scholar] [CrossRef]
- Bai, X.; Chen, Y.-C. Synergistic effect and supernatant nitrogen reduction from anaerobic co-digestion of sewage sludge and pig manure. Bioresour. Technol. Rep. 2020, 10, 100424. [Google Scholar] [CrossRef]
- Hagelqvist, A. Batchwise mesophilic anaerobic co-digestion of secondary sludge from pulp and paper industry and municipal sewage sludge. Waste Manag. 2013, 33, 820–824. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Munaim, M.S.A.; Wahid, Z.B.A. The combined effect of ultrasonic and microwave pre-treatment on bio-methane generation from co-digestion of petrochemical wastewater. J. Clean. Prod. 2017, 145, 303–309. [Google Scholar] [CrossRef]
- Koster, I.W.; Lettinga, G. Anaerobic digestion at extreme ammonia concentrations. Biol. Wastes 1988, 25, 51–59. [Google Scholar] [CrossRef]
- Gai, H.; Jiang, Y.; Qian, Y.; Kraslawski, A. Conceptual design and retrofitting of the coal-gasification wastewater treatment process. Chem. Eng. J. 2008, 138, 84–94. [Google Scholar] [CrossRef]
- Boardman, G.D.; McVeigh, P.J. Use of UASB technology to treat crab processing wastewaters. J. Environ. Eng. 1997, 123, 776. [Google Scholar] [CrossRef]
- Czerwionka, K.; Makinia, J.; Kaszubowska, M.; Majtacz, J.; Angowski, M. Distillery wastes as external carbon sources for denitrification in municipal wastewater treatment plants. Water Sci. Technol. 2012, 65, 1583–1590. [Google Scholar] [CrossRef]
- Tugtas, A.E.; Pavlostathis, S.G. Inhibitory effects of nitrogen oxides on a mixed methanogenic culture. Biotechnol. Bioeng. 2007, 96, 444–455. [Google Scholar] [CrossRef]
- Glanpracha, N.; Annachhatre, A.P. Anaerobic co-digestion of cyanide containing cassava pulp with pig manure. Bioresour. Technol. 2016, 214, 112–121. [Google Scholar] [CrossRef]
- Hu, Y.; Jing, Z.; Sudo, Y.; Niu, Q.; Du, J.; Wu, J.; Li, Y.-Y. Effect of influent COD/SO42− ratios on UASB treatment of a synthetic sulfate-containing wastewater. Chemosphere 2015, 130, 24–33. [Google Scholar] [CrossRef]
- Soto, M.; Méndez, R.; Lema, J.M. Sodium inhibition and sulphate reduction in the anaerobic treatment of mussel processing wastewaters. J. Chem. Technol. Biotechnol. 1993, 58, 1–7. [Google Scholar] [CrossRef]
- Astals, S.; Koch, K.; Weinrich, S.; Hafner, S.D.; Tait, S.; Peces, M. Impact of storage conditions on the methanogenic activity of anaerobic digestion inocula. Water 2020, 12, 1321. [Google Scholar] [CrossRef]
- Holliger, C.; Fruteau de Laclos, H.; Hafner, S.D.; Koch, K.; Weinrich, S.; Astals, S.; Alves, M.; Andrade, D.; Angelidaki, I.; Appels, L.; et al. Requirements for Measurement and Validation of Biochemical Methane Potential (BMP): Standard BMP Methods Document 100, Version 1.4. 2020. Available online: https://www.dbfz.de/en/BMP (accessed on 19 April 2020).
- Bhattarai, S.; Oh, J.-H.; Euh, S.-H.; Krishna Kafle, G.; Hyun Kim, D. Simulation and model validation of sheet and tube type photovoltaic thermal solar system and conventional solar collecting system in transient states. Sol. Energy Mater. Sol. Cells 2012, 103, 184–193. [Google Scholar] [CrossRef]
- Altaş, L. Inhibitory effect of heavy metals on methane-producing anaerobic granular sludge. J. Hazard. Mater. 2009, 162, 1551–1556. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Boe, K.; Angelidaki, I. Anaerobic co-digestion of desugared molasses with cow manure; focusing on sodium and potassium inhibition. Bioresour. Technol. 2011, 102, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- van Houten, R.T.; Lettinga, G. Biological sulphate reduction with synthesis gas: Microbiology and technology. In Progress in Biotechnology; Wijffels, R.H., Buitelaar, R., Bucke, C., Tramper, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 11, pp. 793–799. [Google Scholar] [CrossRef]
- Bajón Fernández, Y.; Soares, A.; Vale, P.; Koch, K.; Masse, A.L.; Cartmell, E. Enhancing the anaerobic digestion process through carbon dioxide enrichment: Initial insights into mechanisms of utilization. Environ. Technol. 2019, 40, 1744–1755. [Google Scholar] [CrossRef]
- Fotidis, I.A.; Karakashev, D.; Kotsopoulos, T.A.; Martzopoulos, G.G.; Angelidaki, I. Effect of ammonium and acetate on methanogenic pathway and methanogenic community composition. FEMS Microbiol. Ecol. 2013, 83, 38–48. [Google Scholar] [CrossRef]
- Ng, W.J. Industrial Wastewater Treatment; Imperial College Press: London, UK, 2006. [Google Scholar]
- Borja, R.; Sánchez, E.; Weiland, P. Influence of ammonia concentration on thermophilic anaerobic digestion of cattle manure in upflow anaerobic sludge blanket (UASB) reactors. Process. Biochem. 1996, 31, 477–483. [Google Scholar] [CrossRef]
- Bujoczek, G.; Oleszkiewicz, J.; Sparling, R.; Cenkowski, S. High Solid Anaerobic Digestion of Chicken Manure. J. Agric. Eng. Res. 2000, 76, 51–60. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Thermophilic anaerobic digestion of livestock waste: The effect of ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Anaerobic thermophilic digestion of manure at different ammonia loads: Effect of temperature. Water Res. 1994, 28, 727–731. [Google Scholar] [CrossRef]
- Abdel Azim, A.; Rittmann, S.K.-M.R.; Fino, D.; Bochmann, G. The physiological effect of heavy metals and volatile fatty acids on Methanococcus maripaludis S2. Biotechnol. Biofuels 2018, 11, 301. [Google Scholar] [CrossRef]
- Shakeri Yekta, S.; Svensson, B.H.; Björn, A.; Skyllberg, U. Thermodynamic modeling of iron and trace metal solubility and speciation under sulfidic and ferruginous conditions in full scale continuous stirred tank biogas reactors. Appl. Geochem. 2014, 47, 61–73. [Google Scholar] [CrossRef]
- Gonzalez-Silva, B.M.; Briones-Gallardo, R.; Razo-Flores, E.; Celis, L.B. Inhibition of sulfate reduction by iron, cadmium and sulfide in granular sludge. J. Hazard. Mater. 2009, 172, 400–407. [Google Scholar] [CrossRef]
- Ahring, B.K.; Westermann, P. Sensitivity of thermophilic methanogenic bacteria to heavy metals. Curr. Microbiol. 1985, 12, 273–276. [Google Scholar] [CrossRef]
- Yue, Z.-B.; Yu, H.-Q.; Wang, Z.-L. Anaerobic digestion of cattail with rumen culture in the presence of heavy metals. Bioresour. Technol. 2007, 98, 781–786. [Google Scholar] [CrossRef]
- Amonette, J.E.; Russell, C.K.; Carosino, K.A.; Robinson, N.L.; Ho, J.T. Toxicity of Al to Desulfovibrio desulfuricans. Appl. Environ. Microbiol. 2003, 69, 4057–4066. [Google Scholar] [CrossRef]
- Cabirol, N.; Barragán, E.J.; Durán, A.; Noyola, A. Effect of aluminium and sulphate on anaerobic digestion of sludge from wastewater enhanced primary treatment. Water Sci. Technol. 2003, 48, 235–240. [Google Scholar] [CrossRef]
- Klüber, H.D.; Conrad, R. Inhibitory effects of nitrate, nitrite, NO and N2O on methanogenesis by Methanosarcina barkeri and Methanobacterium bryantii. FEMS Microbiol. Ecol. 1998, 25, 331–339. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Ahring, B.K. Effects of hydrogen and formate on the degradation of propionate and butyrate in thermophilic granules from an upflow anaerobic sludge blanket reactor. Appl. Environ. Microbiol. 1993, 59, 2546–2551. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Biogas production as affected by heavy metals in the anaerobic digestion of sludge. Egypt. J. Pet. 2014, 23, 409–417. [Google Scholar] [CrossRef]
- Stasinakis, A.S.; Thomaidis, N.S. Fate and Biotransformation of Metal and Metalloid Species in Biological Wastewater Treatment Processes. Crit. Rev. Environ. Sci. Technol. 2010, 40, 307–364. [Google Scholar] [CrossRef]
- Field, J.A.; Sierra-Alvarez, R.; Cortinas, I.; Feijoo, G.; Moreira, M.T.; Kopplin, M.; Gandolfi, A.J. Facile Reduction of Arsenate in Methanogenic Sludge. Biodegradation 2004, 15, 185–196. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2016; Available online: https://iwaponline.com/ebooks/book/286/Coagulation-and-Flocculation-in-Water-and (accessed on 15 September 2020).
- Jeong, T.-Y.; Chung, H.-K.; Yeom, S.H.; Choi, S.S. Analysis of methane production inhibition for treatment of sewage sludge containing sulfate using an anaerobic continuous degradation process. Korean, J. Chem. Eng. 2009, 26, 1319–1322. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ramanujam, T.K. Effect of chloride and condensable tannin in anaerobic degradation of tannery wastewaters. Bioprocess. Eng. 1999, 20, 499. [Google Scholar] [CrossRef]
- Rath, K.M.; Maheshwari, A.; Bengtson, P.; Rousk, J. Comparative Toxicities of Salts on Microbial Processes in Soil. Appl. Environ. Microbiol. 2016, 82, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R. Salt Tolerance in Plants and Microorganisms: Toxicity Targets and Defense Responses; Jeon, C., Ed.; Academic Press: Cambridge, MA, USA, 1996; Volume 165, pp. 1–52. [Google Scholar] [CrossRef]
- Ware, A.; Power, N. Modelling methane production kinetics of complex poultry slaughterhouse wastes using sigmoidal growth functions. Renew. Energy 2017, 104, 50–59. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Use of Inoculum, Water and Percolate as Strategy to Avoid Inhibition on Dry-Batch Anaerobic Digestion of Organic Fraction of Municipal Solid Waste. Waste Biomass Valorization 2021, 13, 227–239. [Google Scholar] [CrossRef]
- Maragkaki, A.E.; Fountoulakis, M.; Kyriakou, A.; Lasaridi, K.; Manios, T. Boosting biogas production from sewage sludge by adding small amount of agro-industrial by-products and food waste residues. Waste Manag. 2018, 71, 605–611. [Google Scholar] [CrossRef]
- McCarty, P.L. Anaerobic waste treatment fundamentals. Public Work 1964, 95, 107–112. [Google Scholar]
- Nielsen, H.B.; Angelidaki, I. Strategies for optimizing recovery of the biogas process following ammonia inhibition. Bioresour. Technol. 2008, 99, 7995–8001. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Parkin, G.F. The Effect of Ammonia on Methane Fermentation Processes. J. Water Pollut. Control. Fed. 1989, 61, 55–59. [Google Scholar]
- Akunna, J.C.; Bizeau, C.; Moletta, R. Nitrate reduction by anaerobic sludge using glucose at various nitrate concentrations: Ammonification, denitrification and methanogenic activities. Environ. Technol. 1994, 15, 41–49. [Google Scholar] [CrossRef]
- Yi, X.-H.; Wan, J.; Ma, Y.; Wang, Y.; Guan, Z.; Jing, D.-D. Structure and Succession of Bacterial Communities of the Granular Sludge during the Initial Stage of the Simultaneous Denitrification and Methanogenesis Process. Water Air Soil Pollut. 2017, 228, 1–21. [Google Scholar] [CrossRef]
- Clarens, M.; Bernet, N.; Delgenès, J.-P.; Moletta, R. Effects of nitrogen oxides and denitrification by Pseudomonas stutzeri on acetotrophic methanogenesis by Methanosarcina mazei. FEMS Microbiol. Ecol. 1998, 25, 271–276. [Google Scholar] [CrossRef]
- Borges, L.I.; López-Vazquez, C.M.; García, H.; van Lier, J.B. Nitrite reduction and methanogenesis in a single-stage UASB reactor. Water Sci. Technol. 2015, 72, 2236–2242. [Google Scholar] [CrossRef]
- Yan, G.; Wang, J.; Guo, S. Anaerobic Biochemical Treatment of Wastewater Containing Highly Concentrated Organic Cyanogen. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 29, 529–535. [Google Scholar] [CrossRef]
- Gijzen, H.J.; Bernal, E.; Ferrer, H. Cyanide toxicity and cyanide degradation in anaerobic wastewater treatment. Water Res. 2000, 34, 2447–2454. [Google Scholar] [CrossRef]
- Li, C.; Fang, H.H.P. Inhibition of heavy metals on fermentative hydrogen production by granular sludge. Chemosphere 2007, 67, 668–673. [Google Scholar] [CrossRef]
- Yu, B.; Lou, Z.; Zhang, D.; Shan, A.; Yuan, H.; Zhu, N.; Zhang, K. Variations of organic matters and microbial community in thermophilic anaerobic digestion of waste activated sludge with the addition of ferric salts. Bioresour. Technol. 2015, 179, 291–298. [Google Scholar] [CrossRef]
- Van Bodegom, P.M.; Scholten, J.C.M.; Stams, A.J.M. Direct inhibition of methanogenesis by ferric iron. FEMS Microbiol. Ecol. 2004, 49, 261–268. [Google Scholar] [CrossRef]
- Colussi, I.; Cortesi, A.; Vedova LDella Gallo, V.; Robles, F.K.C. Start-up procedures and analysis of heavy metals inhibition on methanogenic activity in EGSB reactor. Bioresour. Technol. 2009, 100, 6290–6294. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Shei, S.-H. Heavy metal effects on fermentative hydrogen production using natural mixed microflora. Int. J. Hydrogen Energy 2008, 33, 587–593. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Young, L.Y. Toxicity and anaerobic biodegradability of substituted phenols under methanogenic conditions. Environ. Toxicol. Chem. 1989, 8, 853–862. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Sierra-Alvarez, R.; Cortinas, I.; Yenal, U.; Field, J.A. Methanogenic Inhibition by Arsenic Compounds. Appl. Environ. Microbiol. 2004, 70, 5688–5691. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Janzen, N.; Lens, P.N.L. Selenium oxyanion inhibition of hydrogenotrophic and acetoclastic methanogenesis. Chemosphere 2008, 73, 383–388. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; Banihani, Q.; León, G.; Khatri, C.; Field, J.A.; Sierra-Alvarez, R. Toxicity of fluoride to microorganisms in biological wastewater treatment systems. Water Res. 2009, 43, 3177–3186. [Google Scholar] [CrossRef]










| Feed | Inoculum | |
|---|---|---|
| pH | 6.9 ± 0.7 | 7.5 ± 0.4 |
| COD total (mg COD/L) | 49,657 ± 16,717 | 17,125 ± 6029 |
| COD soluble (mg COD/L) | 6346 ± 4438 | 480 ± 266 |
| TAN (mg-N/L) | 1376 ± 1216 | 825 ± 415 |
| FAN (mg-N/L) | 17 ± 36 | 44 ± 50 |
| Total alkalinity (mg CaCO3/L) | 2785 ± 989 | 5389 ± 1699 |
| Partial alkalinity (mg CaCO3/L) | 199 ± 165 | 3611 ± 754 |
| Intermediate alkalinity (mg CaCO3/L) | 2105 ± 814 | 1504 ± 949 |
| Total solids (TS) (% over sample) | 4.4 ± 1.3 | 2.4 ± 0.7 |
| TS (g/kg over sample) | 44 ± 13 | 24 ± 7 |
| VS (% over TS) | 77.5 ± 2.4 | 64.89 ± 2.2 |
| VS (g/kg over TS) | 775 ± 24 | 648.9 ± 22 |
| Cl (mg/L) | 441 ± 375 | 179 ± 229 |
| NO3 (mg/L) | 134 ± 230 | 4 ± 6 |
| SO4 (mg/L) | 49 ± 47 | 4 ± 8 |
| Na (mg/L) | 2337 ± 3415 | 1119 ± 1345 |
| Al (mg/L) | 9 ± 16 | 3 ± 4 |
| Cu (mg/L) | 2 ± 5 | 4 ± 5 |
| Zn (mg/L) | 31 ± 60 | 8 ± 6 |
| As (mg/L) | 0.3 ± 0.6 | 0.1 ± 0.1 |
| Hg (mg/L) | 1.1 ± 1.7 | 0.3 ± 0.5 |
| Parameter Studied | Chemical Added |
|---|---|
| Ammonia | Ammonium chloride |
| Ammonium sulphate | |
| Zinc | Zinc chloride |
| Zinc sulphate | |
| Copper | Copper chloride |
| Copper sulphate | |
| Aluminium | Aluminium chloride |
| Aluminium sulphate | |
| Aluminium sulphate + NaOH | |
| pH | HCl |
| Mercury | Mercury sulphate |
| Arsenic | Sodium arsenate |
| Chloride | Sodium chloride |
| Sulphate | Sodium sulphate |
| Nitrate | Sodium nitrate |
| Beneficial | Non-Effect | Yield Inhibition | Kinetic Inhibition | Toxic | Unknown | |
|---|---|---|---|---|---|---|
| Chloride (mg/L) | - | <3666 | 3666–4535 | - | - | >4535 |
| Ammonia (mg/L) | 635–1460 | <635 1460–1602 | 1602–3405 | 1602–3405 | - | >3405 |
| Aluminium (mg/L) | - | <309 | 309–607 | 309–607 | - | >607 |
| Nitrate (mg/L) | - | <92 332–427 | 92–332 | 92–427 | - | >427 |
| Zinc (mg/L) | - | <87 | 87–498 | - | >498 | - |
| Sulphate (mg/L) | - | <162 | 162–1466 | - | - | >1466 |
| Copper (mg/L) | - | <7 | 7–501 | 91–198 | 501–804 | - |
| Arsenic (mg/L) | - | <17 | - | 17 | - | >17 |
| Mercury (mg/L) | - | <25 495–920 | 25–495 | 25–495 | - | >920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berzal de Frutos, O.; Götze, M.; Pidou, M.; Bajón Fernández, Y. Anaerobic Co-Digestion of Sewage Sludge and Trade Wastes: Beneficial and Inhibitory Effects of Individual Constituents. Processes 2023, 11, 519. https://doi.org/10.3390/pr11020519
Berzal de Frutos O, Götze M, Pidou M, Bajón Fernández Y. Anaerobic Co-Digestion of Sewage Sludge and Trade Wastes: Beneficial and Inhibitory Effects of Individual Constituents. Processes. 2023; 11(2):519. https://doi.org/10.3390/pr11020519
Chicago/Turabian StyleBerzal de Frutos, Olivia, Martin Götze, Marc Pidou, and Yadira Bajón Fernández. 2023. "Anaerobic Co-Digestion of Sewage Sludge and Trade Wastes: Beneficial and Inhibitory Effects of Individual Constituents" Processes 11, no. 2: 519. https://doi.org/10.3390/pr11020519
APA StyleBerzal de Frutos, O., Götze, M., Pidou, M., & Bajón Fernández, Y. (2023). Anaerobic Co-Digestion of Sewage Sludge and Trade Wastes: Beneficial and Inhibitory Effects of Individual Constituents. Processes, 11(2), 519. https://doi.org/10.3390/pr11020519