Green and Cost-Effective Separation of Cadmium from Base Metals in Chloride Medium with Halide-Loaded Anion Exchanger
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
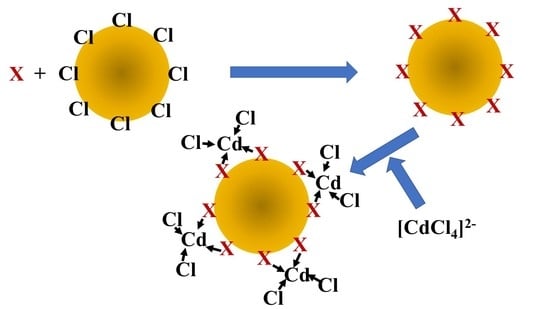
2.2. Preparation of Halide-Loaded Anion Exchangers
2.3. Adsorption Isotherm Study
2.4. Adsorption Kinetics Study
2.5. Effect of Acidity on Cd2+ Adsorption
2.6. Stripping Agent
2.7. Separation Performance
3. Results and Discussion
3.1. Selection of Ligand and Concentration in the Feed Solution
3.2. Adsorption Isotherms and Mechanism
3.3. Adsorption Kinetics
3.3.1. Effect of Contact Time
3.3.2. Pseudo-First Order Kinetics
3.3.3. Pseudo-Second Order Kinetics
3.3.4. Intra-Particle Diffusion Kinetics
3.4. Comparison of Dynamic Retention Efficiency of Cd2+
3.5. Effect of Acidity
3.6. Removal of Retained Foreign Metal Ions
3.7. Stripping of Cd2+
3.8. Separation Performance
3.9. Comparison with Conventional and Reported Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kekesi, T.; Isshiki, M. Ultra high purification of copper chloride solutions by anion exchange. Hydrometallurgy 1997, 45, 345–361. [Google Scholar] [CrossRef]
- Moradkhani, D.; Urbani, M.; Cheng, C.Y.; Askari, M.; Bastani, D. The separation of cadmium from zinc with a synergistic mixture of nonylsalicyclic acid and triisobutylphosphine sulphide. Hydrometallurgy 2005, 78, 129–136. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Bafghi, M.S.; Moradkhani, D.; Ilkhchi, M.K. A review on hydrometallurgical extraction and recovery of cadmium from various resources. Miner. Eng. 2007, 20, 211–220. [Google Scholar] [CrossRef]
- Kouzbour, S.; Gourich, B.; Gros, F.; Vial, C.; Allam, F.; Stiriba, Y. Comparative analysis of industrial processes for cadmium removal from phosphoric acid: A review. Hydrometallurgy 2019, 188, 222–247. [Google Scholar] [CrossRef]
- Mashhadikhan, S.; Amooghin, A.E.; Sanaeepur, H.; Shirazi, M.M.A. A critical review on cadmium recovery from wastewater towards environmental sustainability. Desalination 2022, 535, 115815. [Google Scholar] [CrossRef]
- Blumbergs, E.; Serga, V.; Platacis, E.; Maiorov, M.; Shishkin, A. Cadmium recovery from spent Ni-Cd batteries: A brief review. Metals 2021, 11, 1714. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Lach, J.; Wróbel, K.; Domańska, U. New method for recovery of nickel and cadmium from the ”black mass” of spent Ni-Cd batteries by solvent extraction. J. Mol. Liq. 2022, 357, 119087. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Rosalém, S.F. Electrochemical recovery of cadmium from spent Ni-Cd batteries. J. Power Sources 2005, 139, 366–370. [Google Scholar] [CrossRef]
- Reddy, B.R.; Priya, D.N. Chloride leaching and solvent extraction of cadmium, cobalt and nickel from spent nickel-cadmium batteries using Cyanex 923 and 272. J. Power Sources 2006, 161, 1428–1434. [Google Scholar] [CrossRef]
- Rudnik, E.; Nikiel, M. Hydrometallurgical recovery of cadmium and nickel from spent Ni–Cd batteries. Hydrometallurgy 2007, 89, 61–71. [Google Scholar] [CrossRef]
- Gotfryd, L.; Cox, M. The selective recovery of cadmium(II) from sulfate solutions by a counter-current extraction-stripping process using a mixture of diisopropylsalicyclic acid and Cyanex 471X. Hydrometallurgy 2006, 81, 226–233. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Fu, L.; Zhang, G.; Zuo, Y.; Zhang, L. Mechanism and kinetics analysis of valuable metals leaching from copper-cadmium slag assisted by ultrasound cavitation. J. Clean. Prod. 2022, 379, 134775. [Google Scholar] [CrossRef]
- Han, G.; Wang, J.; Sun, H.; Liu, B.; Huang, Y. A critical review on the removal and recovery of hazardous Cd from Cd-containing secondary resources in Cu-Pb-Zn smelting processes. Metals 2022, 12, 1846. [Google Scholar] [CrossRef]
- Gouvea, L.R.; Morais, C.A. Recovery of zinc and cadmium from industrial waste by leaching/cementation. Miner. Eng. 2007, 20, 956–958. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Bernardes, A.M.; Tenório, J.A.S. An overview on the current processes for the recycling of batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Tenório, J.A.S. Fundamental aspects of recycling of nickel–cadmium batteries through vacuum distillation. J. Power Sources 2004, 135, 320–326. [Google Scholar] [CrossRef]
- Mauchauffée, S.; Meux, E.; Schneider, M. Selective precipitation of cadmium from nickel cadmium sulphate solutions using sodium decanoate. Sep. Purif. Technol. 2008, 62, 394–400. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Penha, T.R.; Sirtoli, S. Chemical and electrochemical recycling of the negative electrodes from spent Ni-Cd batteries. J. Power Sources 2007, 163, 1114–1119. [Google Scholar] [CrossRef]
- Amin, N.K.; El-Ashtoukhy, E.-S.Z.; Abdelwahab, O. Rate of cadmium ions removal from dilute solutions by cementation on zinc using a rotating fixed bed reactor. Hydrometallurgy 2007, 89, 224–232. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Moradkhani, D. The electrowinning of cadmium in the presence of zinc. Hydrometallurgy 2010, 105, 168–171. [Google Scholar] [CrossRef]
- Preston, J.S.; Patrick, J.H.; Steinbach, G. The selective solvent extraction of cadmium by mixtures of carboxylic acids and trialkylphosphine sulphides. Part 2. Practical applications in the separation of cadmium of cadmium from zinc and nickel. Hydrometallurgy 1994, 36, 143–160. [Google Scholar] [CrossRef]
- Gupta, B.; Deep, A.; Malik, P. Extraction and recovery of cadmium using Cyanex 923. Hydrometallurgy 2001, 61, 65–67. [Google Scholar] [CrossRef]
- Itabashi, H.; Yoshida, M.; Kawamoto, H. Kinetically controlled separation of cadmium(II) from zinc(II) with dithizone in the presence of nitrilotriacetic acid. Anal. Sci. 2001, 17, 1301–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, B.R.; Priya, D.N.; Kumar, J.R. Solvent extraction of cadmium (II) from sulphate solutions using TOPS 99, PC 88A, Cyanex 272 and their mixtures. Hydrometallurgy 2004, 74, 277–283. [Google Scholar] [CrossRef]
- Yaftian, M.R.; Zamani, A.A.; Parinejad, M.; Shams, E. Ion-pair extraction of cadmium complex anions from hydrochloric acid media using oxonium ion-dicyclohexyl-18-crown-6 complex. Sep. Purif. Technol. 2005, 42, 175–180. [Google Scholar] [CrossRef]
- Babakhani, A.; Rashchi, F.; Zakeri, A.; Vahidi, E. Selective separation of nickel and cadmium from sulfate solutions of spent nickel–cadmium batteries using mixtures of D2EHPA and Cyanex 302. J. Power Sources 2014, 247, 127–133. [Google Scholar] [CrossRef]
- Lee, L.Y.; Morad, N.; Ismail, N.; Talebi, A.; Rafatullah, M. Optimization for liquid-liquid extraction of Cd(II) over Cu(II) ions from aueous slutions uing inic lquid Aliquat 336 with tibutyl posphate. Int. J. Mol. Sci. 2020, 21, 6860. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Zhang, Q.; Wei, X.; Guo, J. Separation study of cadmium through an emulsion liquid membrane using triisooctylamine as mobile carrier. Talanta 1998, 46, 927–932. [Google Scholar] [CrossRef]
- Choi, D.W.; Kim, Y.H. Cadmium removal using hollow fiber membrane with organic extractant. Korean J. Chem. Eng. 2003, 20, 768–771. [Google Scholar] [CrossRef]
- Swain, B.; Sarangi, K.; Das, R.P. Effect of different anions on the separation of cadmium and zinc by supported liquid membrane using TOPS-99 as mobile carrier. J. Membr. Sci. 2006, 277, 240–248. [Google Scholar] [CrossRef]
- Adelung, S.; Lohrengel, B.; Nghiem, L.D. Selective transport of cadmium by PVC/Aliquat 336 polymer inclusion membranes (PIMs): The role of membrane composition and solution chemistry. Membr. Water Treat. 2012, 3, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Yan, X. An imprinted organic-inorganic hybrid sorbent for selective separation of cadmium from aqueous solution. Anal. Chem. 2004, 76, 453–457. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, Y.; Chang, X.; Chen, S.; Huang, X. Selective solid-phase extraction of trace cadmium(II) with an ionic imprinted polymer prepared from a dual-ligand monomer. Anal. Chim. Acta 2007, 593, 123–128. [Google Scholar] [CrossRef]
- Li, F.; Jiang, H.; Zhang, S. An ion-imprinted silica-supported organic-inorganic hybrid sorbent prepared by a surface imprinting technique combined with a polysaccharide incorporated sol-gel process for selective separation of cadmium(II) from aqueous solution. Talanta 2007, 71, 1487–1493. [Google Scholar] [CrossRef]
- Akama, Y.; Ito, M.; Tanaka, S. Selective separation of cadmium from cobalt, copper, iron(III) and zinc by water-based two-phase system of tetrabutylammonium bromide. Talanta 2000, 53, 645–650. [Google Scholar] [CrossRef]
- Aparajith, B.; Kumar, A.; Hodder, D.; Gupta, M.L. Recovery of cadmium from hydrometallurgical zinc smelter by selective leaching. Hydrometallurgy 2010, 102, 31–36. [Google Scholar] [CrossRef]
- Turan, M.D.; Safarzadeh, M.S. Separation of zinc, cadmium and nickel from ZnO–CdO–NiO mixture through baking with ammonium chloride and leaching. Hydrometallurgy 2012, 119–120, 1–7. [Google Scholar] [CrossRef]
- Dybczyński, R.; Samczyński, Z. The use of amphoteric ion exchange resin for the selective separation of cadmium from other radioelements present in neutron irradiated biological materials. J. Radioanal. Nucl. Chem. 1991, 150, 143–153. [Google Scholar] [CrossRef]
- Fröhlich, S.; Sewing, D. The BATENUS process for recycling mixed battery waste. J. Power Sources 1995, 57, 27–30. [Google Scholar] [CrossRef]
- Ghatas, N.E. Purification of Zinc Sulfate Solutions. Canadian Patent 1,046,288, 25 September 1979. [Google Scholar]
- Saeed, M.M.; Ahmed, M. Retention, kinetics and thermodynamics profile of cadmium and adsorption from iodide medium onto polyurethane foam and its separation from zinc bulk. Anal. Chim. Acta 2004, 525, 289–297. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumar, V.; Jeong, J.; Lee, J.-C. Review on solvent extraction of cadmium from various solutions. Hydrometallurgy 2012, 111–112, 1–9. [Google Scholar] [CrossRef]
- Hadioui, M.; Mecherri, M.O.; Harrache, Z. Predictive simulation for separation of toxic metal ions by ion-exchange resins in complex media. J. Chem. Biochem. Mol. Biol. 2008, 2, 1–15. [Google Scholar]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Strelow, F.W.E. Improved separation of cadmium from indium, zinc, gallium and other elements by anion-exchange chromatography in hydrochloric-nitric acid mixtures. Anal. Chim. Acta 1978, 100, 577–588. [Google Scholar] [CrossRef]
- Kallmann, S.; Oberthin, H.; Liu, R. Determination of cadmium in zinc concentrates and other zinc-rich materials: A cation exchange procedure. Anal. Chem. 1960, 34, 58–60. [Google Scholar] [CrossRef]
- Korkisch, J.; Sorio, A. Determination of cadmium, copper and lead in natural waters after anion-exchange separation. Anal. Chim. Acta 1975, 76, 393–399. [Google Scholar] [CrossRef]
- Fritz, J.S.; Garralda, B.B. Cation exchange separation of metal ions with hydrochloric acid. Anal. Chem. 1962, 34, 102–106. [Google Scholar] [CrossRef]
- Strelow, F.W.E. Separation of cadmium from uranium, cobalt, nickel, manganese, zinc, coppor, titanium, and other elements by cation exchange chromatography. Anal. Chem. 1960, 32, 363–365. [Google Scholar] [CrossRef]
- Kallmann, S.; Oberthin, H.; Lin, R. Determination of cadmium in zinc concentrates and other zinc-rich materials: Anion exchange procedure. Anal. Chem. 1958, 30, 1846–1848. [Google Scholar] [CrossRef]
- Korkisch, J.; Feik, F. Anion-exchange separation of cadmium in organic solvent-nitric acid media. Anal. Chim. Acta 1965, 32, 110–116. [Google Scholar] [CrossRef]
- Patnaik, P. Dean’s Analytical Chemistry Handbook; McGraw-Hill Companies, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Adeloju, S.B.; Zhang, Y. Vapor generation atomic absorption spectrometric determination of cadmium in environmental samples with in-line anion exchange separation. Anal. Chem. 2009, 81, 4249–4255. [Google Scholar] [CrossRef]
- Bartolozzi, M.; Braccini, G.; Bonvini, S.; Marconi, P.F. Hydrometallurgical recovery process for nickel-cadmium spent batteries. J. Power Sources 1995, 55, 247–250. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M.; Citak, D.; Soylak, M. Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J. Hazard. Mater. 2007, 149, 283–291. [Google Scholar] [CrossRef]
- Jain, C.K. Adsorption of zinc onto bed sediments of the River Ganga: Adsorption models and kinetics. Hydrol. Sci. 2001, 46, 419–434. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ onto phosphoric acid modified rice husk. IOSR-J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Eliezer, I.; Moreno, A. Potentiometric study of mixed cadmium halide complexes in aqueous solution. J. Chem. Eng. Data 1974, 19, 226–228. [Google Scholar] [CrossRef]
- Zelić, M.; Branica, M. Voltammetric study of cadmium complexation in halogenide mixtures of high ionic strength. Electroanalysis 1992, 4, 701–706. [Google Scholar] [CrossRef]
- Wołowicz, A.; Wawrzkiewicz, M. Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method. Processes 2021, 9, 285. [Google Scholar] [CrossRef]
- Marcus, Y.; Eliezer, I. The anion exchange of metal complexes-XII. The cadmium and mercury-halide systems. J. Inorg. Nucl. Chem. 1963, 25, 867–874. [Google Scholar] [CrossRef]
- Ünlü, N.; Ersoz, M. Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions. J. Hazard. Mater. 2006, 136, 272–280. [Google Scholar] [CrossRef]
- Farouq, R.; Yousef, N.S. Equilibrium and kinetics studies of adsorption of copper(II) ions on natural biosorbent. Int. J. Chem. Eng. Appl. 2015, 6, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Yang, J.; Zhang, L.; Cao, B.; Liu, L.; Gong, W. Removal of cadmium and lead from aqueous solutions using iron phosphate-modified pollen microspheres as adsorbents. Rev. Adv. Mater. Sci. 2021, 60, 365–376. [Google Scholar] [CrossRef]






| Langmuir model | Ka | qm(mg/g) | R2 | |
| Cl-resin | 0.0045 | 53.19 | 0.9991 | |
| Br-resin | 0.0063 | 54.75 | 0.9972 | |
| I-resin | 0.0277 | 68.97 | 0.9777 | |
| Freundlich model | KF | n | R2 | |
| Cl-resin | 0.5867 | 1.446 | 0.9842 | |
| Br-resin | 0.8734 | 1.581 | 0.9835 | |
| I-resin | 12.325 | 3.671 | 0.9882 |
| Resin | C0 (mg/L) | Pseudo-First Order Parameters | Pseudo-Second Order Parameters | Intra-Particle Diffusion Rate Constants | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe (exp., mg/g) | qe (Theor. mg/g) | k1 (min−1) | R2 | k2 × 103 (g/mg−1 min−1) | qe (Theor., mg/g) | R2 | kid (μg g−1 min−1/2) | R2 | ||
| Cl-resin | 40 | 10.1 | 8.98 | 0.0513 | 0.9792 | 7.3 | 11.7 | 0.9808 | 1.36 | 0.9826 |
| 80 | 21.2 | 18.8 | 0.0508 | 0.9465 | 3.6 | 24.2 | 0.9754 | 2.83 | 0.9798 | |
| Br-resin | 40 | 13.2 | 12.1 | 0.0687 | 0.9968 | 6.7 | 15.2 | 0.9938 | 1.85 | 0.9553 |
| 80 | 24.3 | 20.6 | 0.0642 | 0.9817 | 4.6 | 27.0 | 0.9941 | 3.27 | 0.9448 | |
| I-resin | 40 | 16.8 | 15.5 | 0.0878 | 0.9984 | 6.6 | 19.2 | 0.9983 | 2.40 | 0.9144 |
| 80 | 36.6 | 33.6 | 0.0731 | 0.9951 | 2.5 | 42.4 | 0.9965 | 5.21 | 0.9386 | |
| Ion Exchanger | Cl-Resin | Br-Resin | I-Resin | Cl-Fibre | Br-Fibre | I-Fibre |
|---|---|---|---|---|---|---|
| Retention efficiency (%) | 66.3 | 67.1 | 94.5 | 81.6 | 86.8 | 99.5 |
| Retention efficiency after re-percolating the effluent (%) | 84.3 | 89.3 | 99.1 |
| Element | Element Recovery in Eluate (% of Total Input) | |||||
|---|---|---|---|---|---|---|
| Cl-Resin | Br-Resin | I-Resin | Cl-Fibre | Br-Fibre | I-Fibre | |
| Cd2+ | 24.18 | 24.39 | 24.20 | 63.86 | 61.63 | 49.28 |
| Zn2+ | 1.85 | 1.67 | 0.98 | 0.24 | 0.08 | 0.06 |
| Cu2+ | ND a | ND | NE b | ND | 0.02 | NE |
| Pb2+ | ND | ND | 2.5 | ND | ND | 7.5 |
| Mn2+ | ND | ND | ND | ND | ND | ND |
| Fe3+ | ND | ND | ND | ND | ND | ND |
| Co2+ | ND | ND | ND | ND | ND | ND |
| Ni2+ | ND | ND | ND | ND | ND | ND |
| Element | Element Recovery in Eluate (% of Total Input) | |||||
|---|---|---|---|---|---|---|
| Cl-Resin | Br-Resin | I-Resin | Cl-Fibre | Br-Fibre | I-Fibre | |
| Cd2+ | 35.45 | 37.30 | 67.15 | 60.45 | 54.40 | 68.20 |
| Zn2+ | 2.12 | 1.79 | 1.19 | 0.30 | 0.24 | 0.20 |
| Cu2+ | 0.023 | 0.029 | NE | 0.036 | 0.066 | NE |
| Pb2+ | 0.06 | 0.04 | 0.13 | <0.01 | <0.01 | 0.17 |
| Mn2+ | ND | ND | ND | ND | ND | ND |
| Fe3+ | ND | ND | ND | ND | ND | ND |
| Co2+ | ND | ND | ND | ND | ND | ND |
| Ni2+ | ND | ND | ND | ND | ND | ND |
| En Concentration (M) | Cd2+ Recovery (%) | Zn2+ Recovery (%) | Separation Factor (fCd/Zn) |
|---|---|---|---|
| 0.1 | 71.73 | 0.13 | 5518 |
| 0.2 | 88.45 | 0.04 | 22,113 |
| 0.3 | 97.03 | 0.02 | 48,515 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Duan, X.; Hu, C.; Du, G.; Wang, Y. Green and Cost-Effective Separation of Cadmium from Base Metals in Chloride Medium with Halide-Loaded Anion Exchanger. Processes 2023, 11, 1051. https://doi.org/10.3390/pr11041051
Zhang Y, Duan X, Hu C, Du G, Wang Y. Green and Cost-Effective Separation of Cadmium from Base Metals in Chloride Medium with Halide-Loaded Anion Exchanger. Processes. 2023; 11(4):1051. https://doi.org/10.3390/pr11041051
Chicago/Turabian StyleZhang, Yanlin, Xiaofei Duan, Chaoquan Hu, Guanshang Du, and Yong Wang. 2023. "Green and Cost-Effective Separation of Cadmium from Base Metals in Chloride Medium with Halide-Loaded Anion Exchanger" Processes 11, no. 4: 1051. https://doi.org/10.3390/pr11041051
APA StyleZhang, Y., Duan, X., Hu, C., Du, G., & Wang, Y. (2023). Green and Cost-Effective Separation of Cadmium from Base Metals in Chloride Medium with Halide-Loaded Anion Exchanger. Processes, 11(4), 1051. https://doi.org/10.3390/pr11041051