Modification of Meso-Micromixing Interaction Reaction Model in Continuous Reactors
Abstract
:1. Introduction
2. Modified Meso-Micromixing Interaction Reaction Model
2.1. Initial Mixing-Related Reaction Model
2.2. Comparison of Batch and Continuous Conditions
2.3. Model Modification
3. Methods and Materials
3.1. 3D-Printed Split-and-Recombine Millimeter-Scale Reactor
3.2. Numerical Simulation
3.3. Mixing Performance Experiments
3.4. Data Reduction
3.5. Optimization Procedure
4. Results and Discussion
4.1. Validation of Modified Model Accuracy
4.2. Effect of Parameters on Mixing
4.2.1. Effect of Turn
4.2.2. Effect of Inlet Flow Rates
4.2.3. Effect of Cross-Sectional Area of the Grooves
4.3. Optimization Calculations
4.3.1. Optimization Objectives
4.3.2. Results of Gaussian Process Regression
4.3.3. Results of Bayesian Optimization
5. Conclusions
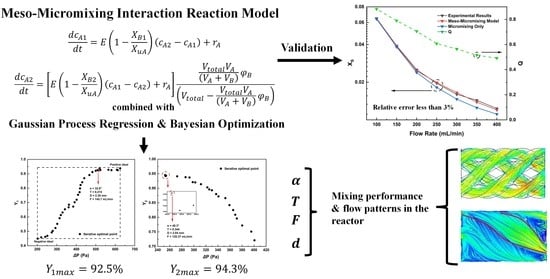
- A modified meso-micromixing interaction reaction model was developed based on the flow characteristics in continuous reactors. The model was validated by comparing experimentally obtained yields with those predicted by this model. The modified model significantly reduced error in predicted product yields from approximately 15% to within 3%, compared to the model containing the micromixing term only.
- Mixing performance in the reactor was improved by characterizing the decreasing XS with increasing flow rate, the degree of twist in the mixing element’s grooves, and the decreasing cross-sectional area of grooves. A high flow rate intensifies the energy dissipation of the fluid in the annular space between two mixing elements; high turn extends the flow path and increases the contact area between the areas with high and low flow velocities in the twisted grooves. When the cross-sectional area in the grooves becomes small, a significant plume flow can be formed in the annular space, improving mixing performance.
- The optimization, in which the yields of target products and pressured drop in the reactors were chosen as the optimization objectives, was based on the modified model and performed by BO along with GPR. We obtained the highest product yield while keeping the pressure drop low. For the intermediate product, the yield was 92.5%, while the pressure drop in the reactor was 510.50 Pa. For the product in the last reaction, the yield was 94.3%, while the pressure drop in the reactor was 253.81 Pa. The corresponding combinations of reactor parameters were obtained. This kind of optimization method can be applied to the design of various reactors, providing a reference for structural selection and operational parameter determination.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Artificial neural network, for short | |
| Bayesian optimization, for short | |
| The mole concentration of component i, mol/m3 | |
| Groove depth, mm | |
| The hydrodynamic diameter of the reactor cross-section, m | |
| The engulfment rate in terms of micromixing, s−1 | |
| Energy dissipation rate, for short | |
| Flow rate, mL/min | |
| Gaussian process regression, for short | |
| Criteria for multi-objective optimization | |
| Distance between two mixing elements, mm | |
| Length of a mixing element, mm | |
| Mean square error, for short | |
| Local pressure field, Pa | |
| Pressure drop in the full domain of reactors | |
| Intrinsic reaction rate of component i, mol/(m3·s) | |
| Outer radius of tube-in-tube reactors, mm | |
| Inner radius of tube-in-tube reactors, mm | |
| Coefficient of determination | |
| Reynold number | |
| Skewness of curved grooves | |
| Split-and-recombine reactor, for short | |
| Mesomixing characteristic time, s | |
| Micromixing characteristic time, s | |
| Local velocity field, m/s | |
| Average velocity along the flow direction, m/s | |
| Volume of the reactor fluid domain | |
| Ratio of initial flow rates in a tubular reactor | |
| Volume of micromixed fluid relative to the whole fluid | |
| Volume fraction which contains the partially segregated fluid as islands, embedded in a sea | |
| Intermediate product yield | |
| Final product yield | |
| Axial position of the reactors, m | |
| Greek symbols | |
| Circulation angle, ° | |
| Energy dissipation rate, m2/s3 | |
| Average from the integral scale of concentration fluctuations to Kolmogorov scale | |
| Dynamic viscosity of the fluid, Pa·s | |
| Kinematic viscosity of the fluid, Pa·s | |
| Density of the fluid, kg/m3 | |
| Ratio of fluid volume change after micromixing | |
References
- Berton, M.; de Souza, J.M.; Abdiaj, I.; McQuade, D.T.; Snead, D.R. Scaling continuous API synthesis from milligram to kilogram: Extending the enabling benefits of micro to the plant. J. Flow Chem. 2020, 10, 73–92. [Google Scholar] [CrossRef]
- Hughes, D.L. Applications of Flow Chemistry in the Pharmaceutical Industry—Highlights of the Recent Patent Literature. Org. Process. Res. Dev. 2020, 24, 1850–1860. [Google Scholar] [CrossRef]
- Nagaki, A.; Yoshida, J.-I. Controlled Polymerization in Flow Microreactor Systems. In Controlled Polymerization and Polymeric Structures: Flow Microreactor Polymerization, Micelles Kinetics, Polypeptide Ordering, Light Emitting Nanostructures; Abe, A., Lee, K.-S., Leiber, L., Kobayashi, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–50. [Google Scholar]
- Parua, S.; Sikari, R.; Sinha, S.; Chakraborty, G.; Mondal, R.; Paul, N.D. Accessing Polysubstituted Quinazolines via Nickel Catalyzed Acceptorless Dehydrogenative Coupling. J. Org. Chem. 2018, 83, 11154–11166. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.L.; Treybal, R.E. Mixing and product distribution for a liquid-phase, second-order, competitive-consecutive reaction. AIChE J. 1971, 17, 718–724. [Google Scholar] [CrossRef]
- Bourne, J.R.; Ravindranath, K.; Thoma, S. Control of product distribution in mixing-controlled reactions. J. Org. Chem. 1988, 53, 5166–5168. [Google Scholar] [CrossRef]
- Rys, P. The Mixing-Sensitive Product Distribution of Chemical Reactions. Chimia 1992, 46, 469. [Google Scholar] [CrossRef]
- Christy, P.F.; Ridd, J.H.; Stears, N.D. Nitration of bibenzyl by nitronium tetrafluoroborate. Comments on the mechanism of nitration by nitronium salts. J. Chem. Soc. B Phys. Org. 1970, 797–801. [Google Scholar] [CrossRef]
- Cox, S.M. Chaotic mixing of a competitive–consecutive reaction. Phys. D Nonlinear Phenom. 2004, 199, 369–386. [Google Scholar] [CrossRef]
- Baldyga, J.; Bourne, J.R.; Dubuis, B.; Etchells, A.W.; Gholap, R.V.; Zimmermann, B. Jet Reactor Scale-Up for Mixing-Controlled Reactions. Chem. Eng. Res. Des. 1995, 73, 497–502. [Google Scholar]
- Bourne, J.R. Mixing and the Selectivity of Chemical Reactions. Org. Process. Res. Dev. 2003, 7, 471–508. [Google Scholar] [CrossRef]
- Bałdyga, J.; Bourne, J.; Hearn, S. Interaction between chemical reactions and mixing on various scales. Chem. Eng. Sci. 1997, 52, 457–466. [Google Scholar] [CrossRef]
- Samant, K.D.; Ng, K.M. Development of liquid-phase agitated reactors: Synthesis, simulation, and scaleup. AIChE J. 1999, 45, 2371–2391. [Google Scholar] [CrossRef]
- Chin, P.; Barney, W.S.; A Pindzola, B. Microstructured reactors as tools for the intensification of pharmaceutical reactions and processes. Curr. Opin. Drug Discov. Dev. 2009, 12, 848–861. [Google Scholar]
- Hessel, V.V.; Gursel, I.V.; Wang, Q.Q.; Noel, T.; Lang, J. Potential Analysis of Smart Flow Processing and Micro Process Technology for Fastening Process Development: Use of Chemistry and Process Design as Intensification Fields. Chem. Eng. Technol. 2012, 35, 1184–1204. [Google Scholar] [CrossRef]
- Movsisyan, M.; Delbeke, E.I.P.; Berton, J.K.E.T.; Battilocchio, C.; Ley, S.V.; Stevens, C.V. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef] [PubMed]
- Morse, P.D.; Beingessner, R.L.; Jamison, T.F. Enhanced Reaction Efficiency in Continuous Flow. Isr. J. Chem. 2017, 57, 218–227. [Google Scholar] [CrossRef]
- Schlüter, M.; Herres-Pawlis, S.; Nieken, U.; Tuttlies, U.; Bothe, D. Small-Scale Phenomena in Reactive Bubbly Flows: Experiments, Numerical Modeling, and Applications. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 625–643. [Google Scholar] [CrossRef]
- Lira, J.O.; Riella, H.G.; Padoin, N.; Soares, C. Computational fluid dynamics (CFD), artificial neural network (ANN) and genetic algorithm (GA) as a hybrid method for the analysis and optimization of micro-photocatalytic reactors: NOx abatement as a case study. Chem. Eng. J. 2022, 431, 133771. [Google Scholar] [CrossRef]
- Gambella, C.; Ghaddar, B.; Naoum-Sawaya, J. Optimization problems for machine learning: A survey. Eur. J. Oper. Res. 2021, 290, 807–828. [Google Scholar] [CrossRef]
- Stulp, F.; Sigaud, O. Many regression algorithms, one unified model: A review. Neural Netw. 2015, 69, 60–79. [Google Scholar] [CrossRef]
- Ryan, E.G.; Drovandi, C.C.; McGree, J.M.; Pettitt, A.N. A Review of Modern Computational Algorithms for Bayesian Optimal Design. Int. Stat. Rev. 2015, 84, 128–154. [Google Scholar] [CrossRef]
- Greenhill, S.; Rana, S.; Gupta, S.; Vellanki, P.; Venkatesh, S. Bayesian Optimization for Adaptive Experimental Design: A Review. IEEE Access 2020, 8, 13937–13948. [Google Scholar] [CrossRef]
- Zuhal, L.R.; Amalinadhi, C.; Dwianto, Y.B.; Palar, P.S.; Shimoyama, K. Benchmarking multi-objective Bayesian global optimization strategies for aerodynamic design. In Proceedings of the 2018 AIAA/ASCE/AHS/ASC Structures, Structural Dynamics, and Materials Conference, Kissimmee, FL, USA, 8–12 January 2018. [Google Scholar]
- Park, S.; Na, J.; Kim, M.; Lee, J.M. Multi-objective Bayesian optimization of chemical reactor design using computational fluid dynamics. Comput. Chem. Eng. 2018, 119, 25–37. [Google Scholar] [CrossRef]
- Guichardon, P.; Falk, L. Characterisation of micromixing efficiency by the iodide–iodate reaction system. Part I: Experimental procedure. Chem. Eng. Sci. 2000, 55, 4233–4243. [Google Scholar] [CrossRef]
- Guichardon, P.; Falk, L.; Villermaux, J. Characterisation of micromixing efficiency by the iodide–iodate reaction system. Part II: Kinetic study. Chem. Eng. Sci. 2000, 55, 4245–4253. [Google Scholar] [CrossRef]
- Gobert, S.R.L.; Kuhn, S.; Braeken, L.; Thomassen, L.C.J. Characterization of Milli- and Microflow Reactors: Mixing Efficiency and Residence Time Distribution. Org. Process. Res. Dev. 2017, 21, 531–542. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, H.; Wang, J.; Yang, Y. Performance Evaluation and Scale-Up Behavior of an Engineered In-Line Mixer for 3D Printing. Ind. Eng. Chem. Res. 2021, 60, 11568–11578. [Google Scholar] [CrossRef]
- Martínez, A.N.M.; Chaudhuri, A.; Assirelli, M.; van der Schaaf, J. Effects of increased viscosity on micromixing in rotor–stator spinning disk reactors. Chem. Eng. J. 2022, 434, 134292. [Google Scholar] [CrossRef]
- Commenge, J.-M.; Falk, L. Villermaux–Dushman protocol for experimental characterization of micromixers. Chem. Eng. Process. Process. Intensif. 2011, 50, 979–990. [Google Scholar] [CrossRef]
- Arian, E.; Pauer, W. A comprehensive investigation of the incorporation model for micromixing time calculation. Chem. Eng. Res. Des. 2021, 175, 296–308. [Google Scholar] [CrossRef]
- Wenzel, D.; Assireli, M.; Rossen, H. On the reactant concentration and the reaction kinetics in the Villermaux-Dushman protocol. Chem. Eng. Process.-Process Intensif. 2018, 130, 332–341. [Google Scholar] [CrossRef]
- Fournier, M.C.; Falk, L.; Villermaux, J. A new parallel competing reaction system for assessing micromixing efficiency—Experimental approach. Chem. Eng. Sci. 1996, 51, 5053–5064. [Google Scholar] [CrossRef]
- Khalde, C.M.; Ramanan, V.; Sangwai, J.S.; Ranade, V.V. Passive Mixer cum Reactor Using Threaded Inserts: Investigations of Flow, Mixing, and Heat Transfer Characteristics. Ind. Eng. Chem. Res. 2020, 59, 3943–3961. [Google Scholar] [CrossRef]
- Baldyga, J.; Bourne, J. A fluid mechanical approach to turbulent mixing and chemical reaction part II micromixing in the light of turbulence theory. Chem. Eng. Commun. 1984, 28, 243–258. [Google Scholar] [CrossRef]
- Bałdyga, J.; Bourne, J. Interactions between mixing on various scales in stirred tank reactors. Chem. Eng. Sci. 1992, 47, 1839–1848. [Google Scholar] [CrossRef]
- Baldyga, J.; Bourne, J. Simplification of micromixing calculations. I. Derivation and application of new model. Chem. Eng. J. 1989, 42, 83–92. [Google Scholar] [CrossRef]
- Bałdyga, J.; Pohorecki, R. Turbulent micromixing in chemical reactors—A review. Chem. Eng. J. Biochem. Eng. J. 1995, 58, 183–195. [Google Scholar] [CrossRef]
- Ghanem, A.; Lemenand, T.; Valle, D.D.; Peerhossaini, H. Static mixers: Mechanisms, applications, and characterization methods—A review. Chem. Eng. Res. Des. 2014, 92, 205–228. [Google Scholar] [CrossRef]
- Baldyga, J.; Bourne, J.; Yang, Y. Influence of feed pipe diameter on mesomixing in stirred tank reactors. Chem. Eng. Sci. 1993, 48, 3383–3390. [Google Scholar] [CrossRef]
- Villermaux, J.; Falk, L. A generalized mixing model for initial contacting of reactive fluids. Chem. Eng. Sci. 1994, 49, 5127–5140. [Google Scholar] [CrossRef]
- Ertesvåg, I.S.; Magnussen, B.F. The Eddy Dissipation Turbulence Energy Cascade Model. Combust. Sci. Technol. 2000, 159, 213–235. [Google Scholar] [CrossRef]
- Martínez, A.N.M.; Jansen, R.; Walker, K.; Assirelli, M.; van der Schaaf, J. Experimental and modeling study on meso- and micromixing in the rotor–stator spinning disk reactor. Chem. Eng. Res. Des. 2021, 173, 279–288. [Google Scholar] [CrossRef]
- Woldemariam, M.; Filimonov, R.; Purtonen, T.; Sorvari, J.; Koiranen, T.; Eskelinen, H. Mixing performance evaluation of additive manufactured milli-scale reactors. Chem. Eng. Sci. 2016, 152, 26–34. [Google Scholar] [CrossRef]
- Shields, B.J.; Stevens, J.; Li, J.; Parasram, M.; Damani, F.; Alvarado, J.I.M.; Janey, J.M.; Adams, R.P.; Doyle, A.G. Bayesian reaction optimization as a tool for chemical synthesis. Nature 2021, 590, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, Z.; Liu, W. Active design for the tube insert of center-connected deflectors based on the principle of exergy destruction minimization. Int. J. Heat Mass Transf. 2020, 150, 119260. [Google Scholar] [CrossRef]
- Ofman, P.; Struk-Sokołowska, J. Artificial Neural Network (ANN) Approach to Modelling of Selected Nitrogen Forms Removal from Oily Wastewater in Anaerobic and Aerobic GSBR Process Phases. Water 2019, 11, 1594. [Google Scholar] [CrossRef]
- Abdollahi, A.; Shams, M. Optimization of shape and angle of attack of winglet vortex generator in a rectangular channel for heat transfer enhancement. Appl. Therm. Eng. 2015, 81, 376–387. [Google Scholar] [CrossRef]
- Neal, R.M. Bayesian Learning for Neural Networks; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 118. [Google Scholar]











| , ° | |||
|---|---|---|---|
| 15, 30, 45, 60, 75 | 0.5, 1, 1.5, 2, 2.5 | 0.2, 0.4, 0.6, 0.8, 1 | 100, 150, 200, 250, 300, 350, 400 |
| Materials | Concentration [mol/L] |
|---|---|
| H2BO3 | 0.09 |
| NaOH | 0.09 |
| KIO3 | 0.006 |
| KI | 0.032 |
| H2SO4 | 0.0026 |
| Information about the Reactions |
|---|
| |
| GPR | ANN | |||
|---|---|---|---|---|
| 1.40880 × 10−4 | 0.94593 | 8.51216 × 10−5 | 0.99670 | |
| 3.38565 × 10−3 | 0.78948 | 2.87026 × 10−4 | 0.97674 | |
| 3.85881 × 10−3 | 0.96288 | 1.45477 × 10−4 | 0.99972 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Yang, N.; Liu, H.; Tang, J.; Wang, C.; Wang, R.; Yang, X. Modification of Meso-Micromixing Interaction Reaction Model in Continuous Reactors. Processes 2023, 11, 1576. https://doi.org/10.3390/pr11051576
Jiang J, Yang N, Liu H, Tang J, Wang C, Wang R, Yang X. Modification of Meso-Micromixing Interaction Reaction Model in Continuous Reactors. Processes. 2023; 11(5):1576. https://doi.org/10.3390/pr11051576
Chicago/Turabian StyleJiang, Junan, Ning Yang, Hanyang Liu, Jianxin Tang, Chenfeng Wang, Rijie Wang, and Xiaoxia Yang. 2023. "Modification of Meso-Micromixing Interaction Reaction Model in Continuous Reactors" Processes 11, no. 5: 1576. https://doi.org/10.3390/pr11051576
APA StyleJiang, J., Yang, N., Liu, H., Tang, J., Wang, C., Wang, R., & Yang, X. (2023). Modification of Meso-Micromixing Interaction Reaction Model in Continuous Reactors. Processes, 11(5), 1576. https://doi.org/10.3390/pr11051576