Influence of Rapeseed Oil on Extruded Plant-Based Meat Analogues: Assessing Mechanical and Rheological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extrusion Process
2.3. Mechanical Measurements
- E = Young’s modulus;
- F = measured force;
- A = probe’s cross-sectional area;
- ε = strain;
- ε was calculated as shown in Equation (2).
- Δl = change in the height of the sample caused by the applied stress;
- l0 = samples’ initial height.
2.4. Rheological Measurements
2.5. Visual Inspection
2.6. Scanning Electron Microscopy
2.7. Encapsulation Efficiency
- = total oil mass in the sample
- = mass of the extracted surface oil
2.8. Interfacial Tension
2.9. Statistical Analysis
3. Results and Discussion
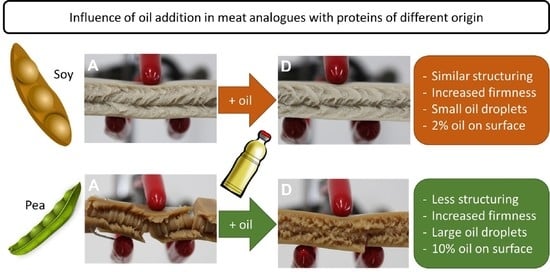
3.1. Effect of Oil on Macroscopic and Microscopic Structure
3.2. Influence of Oil on the Rheological and Mechanical Properties of Meat Analogues
3.3. Encapsulation Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Djekic, I. Environmental Impact of Meat Industry—Current Status and Future Perspectives. Procedia Food Sci. 2015, 5, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Smil, V. Feeding the World: A Challenge for the Twenty-First Century; MIT Press: Cambrige, MA, USA, 2000. [Google Scholar]
- Cheftel, J.C.; Kitagawa, M.; Quéguiner, C. New protein texturization processes by extrusion cooking at high moisture levels. Food Rev. Int. 1992, 8, 235–275. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B. Thermoplastic extrusion-the mechanism of the formation of extrudate structure and properties. J. Am. Oil Chem. Soc. 1993, 70, 414–424. [Google Scholar] [CrossRef]
- Beniwal, A.S.; Singh, J.; Kaur, L.; Hardacre, A.; Singh, H. Meat analogs: Protein restructuring during thermomechanical processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1221–1249. [Google Scholar] [CrossRef]
- Manski, J.M.; van der Goot, A.J.; Boom, R.M. Formation of Fibrous Materials from Dense Calcium Caseinate Dispersions. Biomacromolecules 2007, 8, 1271–1279. [Google Scholar] [CrossRef]
- Dijkstra, M.J.J.; Fokkink, W.J.; Heringa, E.; van Dijk, E.; Abeln, S. The characteristics of molten globule states and folding pathways strongly depend on the sequence of a protein. Mol. Phys. 2018, 116, 3173–3180. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Zhang, B.; Wei, Y. Effects of the specific mechanical energy on the physicochemical properties of texturized soy protein during high-moisture extrusion cooking. J. Food Eng. 2014, 121, 32–38. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Emin, M.A.; Schuchmann, H.P. Process conditions influencing wheat gluten Process conditions influencing wheat gluten extrusion of meat analog products. J. Food Eng. 2017, 198, 28–38. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High Moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Wittek, P.; Zeiler, N.; Karbstein, H.P.; Emin, M.A. High Moisture Extrusion of Soy Protein: Investigations on the Formation of Anisotropic Product Structure. Foods 2021, 10, 102. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B.; Ojokoh, A.O. System parameters and product properties response of soybean protein extruded at wide moisture range. J. Food Eng. 2010, 96, 208–213. [Google Scholar] [CrossRef]
- Guy, R. Extrusion Cooking: Technologies and Applications; Woodhead Publishing: Cambridge, UK, 2001. [Google Scholar]
- Hoek, A.C.; Luning, P.A.; Weijzen, P.; Engels, W.; Kok, F.J.; de Graaf, C. Replacement of meat by meat substitutes. A survey on person- and product-related factors in consumer acceptance. Appetite 2011, 56, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ahn, D.U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Zhao, X.; Sun, P.; Zhao, D.; Jiang, L.; Sui, X. The texture of plant protein-based meat analogs by high moisture extrusion: A review. J. Texture Stud. 2022, 54, 351–364. [Google Scholar] [CrossRef]
- Ilo, S.; Schoenlecher, R.; Berghofe, E. Role of lipids in the extrusion cooking processes. Grasas Y Aceites 2000, 51, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Opaluwa, C.; Lott, T.; Karbstein, H.P.; Emin, M.A. Encapsulation of oil in the high moisture extrusion of wheat gluten: Interrelation between process parameters, matrix viscosity and oil droplet size. Future Foods 2023, 7, 100222. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Y.; Jia, F.; Chen, D.; Zhang, X.; Wang, Q.; Wang, J. Effect of extrusion temperature on the protein aggregation of wheat gluten with the addition of peanut oil during extrusion. Int. J. Biol. Macromol. 2021, 166, 1377–1386. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Chen, Y.; Zhang, X.; Wang, Q.; Chen, D.; Zhang, C. Effect of oil contents on gluten network during the extrusion processing. Czech J. Food Sci. 2019, 37, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Kendler, C.; Duchardt, A.; Karbstein, H.P.; Emin, M.A. Effect of Oil Content and Oil Addition Point on the Extrusion Processing of Wheat Gluten-Based Meat Analogues. Foods 2021, 10, 697. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Wang, Q. Effect of fatty acid saturation degree on the rheological properties of pea protein and its high-moisture extruded product quality. Food Chem. 2022, 390, 133139. [Google Scholar] [CrossRef]
- Liu, K.; Stieger, M.; van der Linden, E.; van de Velde, F. Fat droplet characteristics affect rheological, tribological and sensory properties of food gels. Food Hydrocoll. 2015, 44, 244–259. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Bühler, J.M.; Karbstein, H.P.; Emin, M.A. High moisture extrusion of soy protein concentrate: Influence of thermomechanical treatment on protein-protein interactions and rheological properties. J. Food Eng. 2019, 251, 11–18. [Google Scholar] [CrossRef]
- Gwiazda, S.; Noguchi, A.; Saio, K. Microstructural Studies of Texturized Vegetable Protein Products: Effects of Oil Addition and Transformation of Raw Materials in Various Sections of a Twin Screw Extruder. Food Struct. 1987, 6, 57–61. [Google Scholar]
- Malone, M.E.; Appelqvist, I.A.M.; Norton, I.T. Oral behaviour of food hydrocolloids and emulsions. Part 1. Lubrication and deposition considerations. Food Hydrocoll. 2003, 17, 763–773. [Google Scholar] [CrossRef]
- Daget, N.; Joerg, M.; Bourne, M. Creamy Perception I: In model dessert creams. J. Texture Stud. 1987, 18, 367–388. [Google Scholar] [CrossRef]
- Chen, J.; Dickinson, E. Viscoelastic Properties of Heat-Set Whey Protein Emulsion Gels. J. Texture Stud. 1998, 29, 285–304. [Google Scholar] [CrossRef]
- Chen, J.; Dickinson, E. Viscoelastic Properties of Protein-Stabilized Emulsions: Effect of Protein-Surfactant Interactions. J. Agric. Food Chem. 1998, 46, 91–97. [Google Scholar] [CrossRef]
- Wiedenmann, V.; Oehlkea, K.; van der Schaaf, U.; Hetzera, B.; Greinera, R. Impact of the incorporation of solid lipid nanoparticles on β-lactoglobulin gel matrices. Food Hydrocoll. 2018, 84, 498–507. [Google Scholar] [CrossRef]
- Saavedra Isusi, G.I.; Paz Puga, D.; van der Schaaf, U. Texturing Fermented Emulsion Gels from Soy Protein: Influence of the Emulsifying Agent—Soy Protein vs. Pectin Microgels—On Gel Microstructure, Rheology and Tribology. Foods 2022, 11, 294. [Google Scholar] [CrossRef]
- Willats, W.G.; Orfila, C.; Limberg, G.; Buchholt, H.C.; van Alebeek, G.J.W.M.; Voragen, A.G.; Marcus, S.E.; Christensen, T.M.I.E.; Mikkelsen, J.D.; Murray, B.S.; et al. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties and cell adhesion. J. Biol. Chem. 2001, 276, 19404–19413. [Google Scholar] [CrossRef] [Green Version]
- Fraeye, I.; Colle, I.; Vandevenne, E.; Duvetter, T.; van Buggenhout, S.; Moldenaers, P.; van Loey, A.; Hendrickx, M. Influence of pectin structure on texture of pectin-calcium gels. Innov. Food Sci. Emerg. Technol. 2010, 11, 401–409. [Google Scholar] [CrossRef]
- Tan, L.H.; Chan, L.W.; Heng, P.W.S. Effect of oil loading on microspheres produced by spray drying. J. Microencapsul. 2005, 22, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, J.M.; Campanella, O. Extrusion Processing Technology: Food and Non-Food Biomaterials; John Wiley & Sons Inc.: Chichester, UK, 2014. [Google Scholar]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef]
- McClements, D.J. Protein-stabilized emulsion. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Van Vliet, T. Rheological properties of filled gels. Influence of filler matrix interaction. Colloid Polym. Sci. 1988, 266, 518–524. [Google Scholar] [CrossRef]
- Adams, S.; Frith, W.J.; Stokes, J.R. Influence of particle modulus on the rheological properties of agar microgel suspensions. J. Rheol. 2004, 48, 1195–1213. [Google Scholar] [CrossRef]
- Picout, D.R.; Ross-Murphy, S.B. Rheology of Biopolymer Solutions and Gels. Sci. World J. 2003, 3, 105–121. [Google Scholar] [CrossRef]
- Treolar, L.R.G. The Physics of Rubber Elasticity, 3rd ed; Oxford University Press: Oxford, UK; Clarendon Press: Oxford, UK, 2009. [Google Scholar]
- Ningtyas, D.W.; Tam, B.; Bhandari, B.; Prakash, S. Effect of different types and concentrations of fat on the physico-chemical properties of soy protein isolate gel. Food Hydrocoll. 2021, 111, 106226. [Google Scholar] [CrossRef]
- Pang, Z.; Luo, Y.; Li, B.; Zhang, M.; Liu, X. Effect of different hydrocolloids on tribological and rheological behaviors of soymilk gels. Food Hydrocoll. 2020, 101, 105558. [Google Scholar] [CrossRef]
- Gu, X.; Campbell, L.J.; Euston, S.R. Effects of different oils on the properties of soy protein isolate emulsions and gels. Food Res. Int. 2009, 42, 925–932. [Google Scholar] [CrossRef]
- Van Vliet, T.; Walstra, P. Large deformation and fracture behaviour of gels. Faraday Discuss. 1995, 101, 359–370. [Google Scholar] [CrossRef]
- Sala, G.; van Vliet, T.; Cohen Stuart, M.A.; van Aken, G.A.; van de Velde, F. Deformation and fracture of emulsion-filled gels: Effect of oil content and deformation speed. Food Hydrocoll. 2009, 23, 1381–1393. [Google Scholar] [CrossRef]
- Cuq, B.; Boutrot, F.; Redl, A.; Lullien-Pellerin, V. Study of the Temperature Effect on the Formation of Wheat Gluten Network: Influence on Mechanical Properties and Protein Solubility. J. Agric. Food Chem. 2000, 48, 2954–2959. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, O.H.; Fleming, P.D. A new attempt to reconcile the statistical and phenomenological theories of rubber elasticity. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 1919–1931. [Google Scholar] [CrossRef]










| Sample’s Name | Protein Content | Added Oil Content | Added Water Content |
|---|---|---|---|
| PPI 0% | 45% PPI | 0% | 55% |
| PPI 2% | 44% PPI | 2% | 54% |
| PPI 4% | 43% PPI | 4% | 53% |
| PPI 6% | 42% PPI | 6% | 52% |
| SPC 0% | 54% SPC | 0% | 46% |
| SPC 2% | 53% SPC | 2% | 45% |
| SPC 4% | 52% SPC | 4% | 44% |
| SPC 6% | 51% SPC | 6% | 43% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saavedra Isusi, G.I.; Pietsch, V.; Beutler, P.; Hoehne, S.; Leister, N. Influence of Rapeseed Oil on Extruded Plant-Based Meat Analogues: Assessing Mechanical and Rheological Properties. Processes 2023, 11, 1871. https://doi.org/10.3390/pr11071871
Saavedra Isusi GI, Pietsch V, Beutler P, Hoehne S, Leister N. Influence of Rapeseed Oil on Extruded Plant-Based Meat Analogues: Assessing Mechanical and Rheological Properties. Processes. 2023; 11(7):1871. https://doi.org/10.3390/pr11071871
Chicago/Turabian StyleSaavedra Isusi, Gabriela Itziar, Valerie Pietsch, Philipp Beutler, Sebastian Hoehne, and Nico Leister. 2023. "Influence of Rapeseed Oil on Extruded Plant-Based Meat Analogues: Assessing Mechanical and Rheological Properties" Processes 11, no. 7: 1871. https://doi.org/10.3390/pr11071871
APA StyleSaavedra Isusi, G. I., Pietsch, V., Beutler, P., Hoehne, S., & Leister, N. (2023). Influence of Rapeseed Oil on Extruded Plant-Based Meat Analogues: Assessing Mechanical and Rheological Properties. Processes, 11(7), 1871. https://doi.org/10.3390/pr11071871