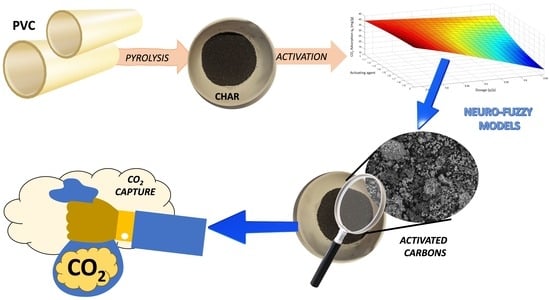
Recycling PVC Waste into CO2 Adsorbents: Optimizing Pyrolysis Valorization with Neuro-Fuzzy Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. PVC-Based Char Production
2.2. PVC-Based Char Activation
2.3. Activated Carbon Characterization
2.4. CO2 Adsorption Tests
2.5. Mathematical Modeling of CO2 Adsorption: Factorial Design
- k, number of studied variables.
- nc, number of central points.
- p, constant for values of k < 5 (p = 0).
Mathematical Modeling of CO2 Adsorption: Neuro-Fuzzy Models
3. Results and Discussion
3.1. CO2 Adsorption Tests
3.2. N2 Adsorption/Desorption Isotherms
3.3. Characterization of PVC-Based AC
3.4. Optimization of the CO2 Adsorption Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plastic Europe. Plastics—The facts 2022. PlasticEurope 2022, 1, 1–17. [Google Scholar]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal Degradation of PVC: A Review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Jiang, S.; Hou, H. Carbonization: A feasible route for reutilization of plastic wastes. Sci. Total Environ. 2020, 710, 136250. [Google Scholar] [CrossRef]
- Scott, D.S.; Czernik, S.R.; Piskorz, J.; Radlein, D.S.A. Fast pyrolysis of plastic wastes. Energy Fuels 1990, 4, 407–411. [Google Scholar] [CrossRef]
- Kunwar, B.; Chen, H.N.; Chandrashekaran, S.; Sharma, B. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Zhang, H.; Pap, S.; Taggart, M.A.; Boyd, K.G.; James, N.A.; Gibb, S.W. A review of the potential utilisation of plastic waste as adsorbent for removal of hazardous priority contaminants from aqueous environments. Environ. Pollut. 2020, 258, 113698. [Google Scholar] [CrossRef]
- Peréz-Huertas, S.; Calero, M.; Ligero, A.; Pérez, A.; Terpiłowski, K.; Martín-Lara, M.A. On the use of plastic precursors for preparation of activated carbons and their evaluation in CO2 capture for biogas upgrading: A review. Waste Manag. 2023, 161, 116–141. [Google Scholar] [CrossRef]
- PlasticsEurope EP. Plastics—The Facts 2019. An Analysis of European Plastics Production, Demand and Waste Data; PlasticEurope: Bruxelles, Belgium, 2019. [Google Scholar]
- Miliute-Plepiene, J.; Fråne, A.; Almasi, A.M. Overview of polyvinyl chloride (PVC) waste management practices in the Nordic countries. Clean. Eng. Technol. 2021, 4, 100246. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.L.; Shao, L.M.; Lü, F.; He, P.J. Upcycling of PET waste into methane-rich gas and hierarchical porous carbon for high-performance supercapacitor by autogenic pressure pyrolysis and activation. Sci. Total Environ. 2021, 772, 145309. [Google Scholar] [CrossRef] [PubMed]
- Kalargaris, I.; Tian, G.; Gu, S. The utilisation of oils produced from plastic waste at different pyrolysis temperatures in a DI diesel engine. Energy 2017, 131, 179–185. [Google Scholar] [CrossRef]
- Yao, D.; Wang, C.H. Pyrolysis and in-line catalytic decomposition of polypropylene to carbon nanomaterials and hydrogen over Fe- and Ni-based catalysts. Appl. Energy 2020, 265, 114819. [Google Scholar] [CrossRef]
- Saeaung, K.; Phusunti, N.; Phetwarotai, W.; Assabumrungrat, S.; Cheirsilp, B. Catalytic pyrolysis of petroleum-based and biodegradable plastic waste to obtain high-value chemicals. Waste Manag. 2021, 127, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Buekens, A.; Cen, K. Waste incineration, PVC, and dioxins. J. Mater. Cycles Waste Manag. 2011, 13, 190–197. [Google Scholar] [CrossRef]
- Ye, L.; Li, T.; Hong, L. Understanding Enhanced Char Formation in the Thermal Decomposition of PVC Resin: Role of Intermolecular Chlorine Loss. Mater. Today Commun. 2021, 26, 102186. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J.J. Pyrolysis of plastic waste: Opportunities and challenges. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Liu, T.; Zhou, Y.; Li, Z.; Deng, S.; Li, Y.; Liang, P. Synergistic removal of HCl and Hg0 in pyrolytic waste plastic gas on Ca and Co loaded carbon aerogel at room temperature. Fuel Process. Technol. 2022, 238, 107497. [Google Scholar] [CrossRef]
- Sharuddin, S.; Abnisa, F.; Daud, W.; Aroua, M. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Zheng, J.; Suh, S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Chang. 2019, 9, 374–378. [Google Scholar] [CrossRef]
- Almroth, B.C.; Eggert, H. Marine Plastic Pollution: Sources, Impacts, and Policy Issues. Rev. Environ. Econ. Policy 2019, 13, 317–326. [Google Scholar] [CrossRef]
- Ligero, A.; Calero, M.; Pérez, A.; Solís, R.R.; Muñoz-Batista, M.J.; Martín-Lara, M.A. Low-cost activated carbon from the pyrolysis of post-consumer plastic waste and the application in CO2 capture. Process Saf. Environ. 2023, 173, 558–566. [Google Scholar] [CrossRef]
- Calero, M.; Iáñez-Rodríguez, I.; Pérez, A.; Martín-Lara, M.A.; Blázquez, G. Neural Fuzzy Modelization of Copper Removal from Water by Biosorption in Fixed-Bed Columns Using Olive Stone and Pinion Shell. Bioresour Technol. 2018, 252, 100–109. [Google Scholar] [CrossRef]
- Singh, J.; Basu, S.; Bhunia, H. Dynamic CO2 adsorption on activated carbon adsorbents synthesized from polyacrylonitrile (PAN): Kinetic and isotherm studies. Microporous Mesoporous Mater. 2019, 280, 357–366. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, J.; Gupta, R.K.; Bhunia, H. Porous carbons derived from polyethylene terephthalate (PET) waste for CO2 capture studies. J. Environ. Manag. 2019, 242, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Choma, J.; Marszewski, M.; Osuchowski, L.; Jagiello, J.; Dziura, A.; Jaroniec, M. Adsorption Properties of Activated Carbons Prepared from Waste CDs and DVDs. ACS Sustain. Chem. Eng. 2015, 3, 733–742. [Google Scholar] [CrossRef]
- Yuan, X.; Lee, J.G.; Yun, H.; Deng, S.; Kim, Y.J.; Lee, J.E.; Kwak, S.K.; Lee, K.B. Solving two environmental issues simultaneously: Waste polyethylene terephthalate plastic bottle-derived microporous carbons for capturing CO2. Chem. Eng. J. 2020, 397, 125350. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B.; Zhu, L. Comparative study on composition, structure, and adsorption behavior of activated carbons derived from different synthetic waste polymers. J. Colloid Interface Sci. 2011, 360, 725–730. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Carbon dioxide adsorption performance of ultramicroporous carbon derived from poly(vinylidene fluoride). J. Anal. Appl. Pyrol. 2014, 106, 147–151. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, B.; Qiao, Y.; Zhang, J.; Wang, W.; Yao, H.; Yu, Y.; Xu, M. Understanding the Pyrolysis Mechanism of Polyvinylchloride (PVC) by Characterizing the Chars Produced in a Wire-Mesh Reactor. Fuel 2016, 166, 526–532. [Google Scholar] [CrossRef]
- Calero, M.; Solís, R.R.; Muñoz-Batista, M.J.; Pérez, A.; Blázquez, G.; Martín-Lara, M.Á. Oil and gas production from the pyrolytic transformation of recycled plastic waste: An integral study by polymer families. Chem. Eng. Sci. 2023, 271, 118569. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Islam, I.; Sultana, S.; Kumer Ray, S.; Parvin Nur, H.; Hossain, M.T.; Md. Ajmotgir, W. Electrical and Tensile Properties of Carbon Black Reinforced Polyvinyl Chloride Conductive Composites. C 2018, 4, 15. [Google Scholar] [CrossRef]
- Rajendran, S.; Uma, T. Effect of ZrO2 on conductivity of PVC–LiBF4–DBP polymer electrolytes. Mater. Lett. 2000, 44, 208–214. [Google Scholar] [CrossRef]
- Pandey, M.; Joshi, G.M.; Mukherjee, A.; Thomas, P. Electrical Properties and Thermal Degradation of Poly(Vinyl Chloride)/Polyvinylidene Fluoride/ZnO Polymer Nanocomposites. Polym. Int. 2016, 65, 1098–1106. [Google Scholar] [CrossRef]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Martins, A.C.; Silva, T.L.; Santos, O.; Visentainer, J.V.; Almeida, V.C. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2016, 288, 778–788. [Google Scholar] [CrossRef]
- Ravikumar, K.; Krishnan, S.; Ramalingam, S.; Balu, K. Optimization of process variables by the application of response surface methodology for dye removal using a novel adsorbent. Dyes Pigm. 2007, 72, 66–74. [Google Scholar] [CrossRef]




| Experiment No. | Activation | Temperature °C | Ratio | Code |
|---|---|---|---|---|
| 1 | NaOH | 760 | 1:1 | 0, −1, 0 |
| 2 * | NaOH | 800 | 1:1 | 0, 0, 0 |
| 3 | NaOH | 800 | 1:2 | 0, 0, 1 |
| 4 | NaOH | 800 | 2:1 | 0, 0, −1 |
| 5 | NaOH | 840 | 1:1 | 0, 1, 0 |
| 6 | KOH | 760 | 2:1 | 1, −1, −1 |
| 7 | KOH | 760 | 1:2 | 1, −1, 1 |
| 8 | KOH | 800 | 1:1 | 1, 0, 0 |
| 9 | KOH | 840 | 1:2 | 1, 1, 1 |
| 10 | KOH | 840 | 2:1 | 1, 1, −1 |
| 11 | N2 | 760 | - | −1, −1 |
| 12 | N2 | 800 | - | −1, 0 |
| 13 | N2 | 840 | - | −1, 1 |
| 14 * | NaOH | 800 | 1:1 | 0, 0, 0 |
| 15 * | NaOH | 800 | 1:1 | 0, 0, 0 |
| Act. Agent | Act. Temperature | Ratio (A/C) | CO2 Uptake (mg/g) |
|---|---|---|---|
| - | - | - | 5.1 |
| NaOH | 760 | 1:1 | 37.3 |
| NaOH | 800 | 1:1 | 37.4 |
| NaOH | 800 | 1:2 | 32.7 |
| NaOH | 800 | 2:1 | 36.4 |
| NaOH | 840 | 1:1 | 35.3 |
| KOH | 760 | 2:1 | 45.6 |
| KOH | 760 | 1:2 | 14.8 |
| KOH | 800 | 1:1 | 37.6 |
| KOH | 840 | 1:2 | 22.7 |
| KOH | 840 | 2:1 | 35.1 |
| N2 | 760 | - | 26.4 |
| N2 | 800 | - | 11.7 |
| N2 | 840 | - | 6.4 |
| Sample | SBET m2/g | VTOTAL cm3/g | VMICRO cm3/g | AMICRO m2/g | MicroPDIAMETER nm |
|---|---|---|---|---|---|
| Char | 3.5 | 0.015 | 0.000 | 0.0 | 0.998 |
| Na-760-1:1 | 152.3 | 0.113 | 0.056 | 112.4 | 0.812 |
| Na-800-1:1 | 135.0 | 2.831 | 0.043 | 92.1 | 0.791 |
| Na-800-1:2 | 82.0 | 0.077 | 0.026 | 50.7 | 0.766 |
| Na-800-2:1 | 46.3 | 0.071 | 0.005 | 10.4 | 0.831 |
| Na-840-1:1 | 79.8 | 0.077 | 0.024 | 47.2 | 0.786 |
| K-760-2:1 | 886.2 | 0.531 | 0.252 | 528.2 | 0.824 |
| K-760-1:2 | 117.8 | 0.093 | 0.035 | 76.9 | 0.840 |
| K-800-1:1 | 530.6 | 0.302 | 0.214 | 447.0 | 0.818 |
| K-840-1:2 | 123.8 | 2.764 | 0.033 | 70.4 | 0.789 |
| K-840-2:1 | 461.5 | 0.297 | 0.192 | 371.8 | 0.859 |
| N2-760 | 7.2 | 0.028 | 0.000 | 0.0 | 0.884 |
| N2-800 | 7.4 | 0.020 | 0.000 | 0.0 | 0.817 |
| N2-840 | 23.8 | 0.050 | 0.000 | 0.0 | 0.825 |
| Sample | Element (%) | |||
|---|---|---|---|---|
| N | C | H | O | |
| Char | 0 | 19.05 | 1.75 | 79.20 |
| Na-800-1:1 | 0.08 | 42.32 | 1.12 | 56.48 |
| K-760-2:1 | 0 | 48.36 | 0.98 | 50.66 |
| Sample | Activation (A) | Act. Temperature °C (T) | Ratio (D) | CO2 Uptake mg/g (qe) | CO2 Uptake Model mg/g (ye) | Error |
|---|---|---|---|---|---|---|
| Na-760-1:1 | 1 | 760 | 0.5 | 37.29 | 37.28 | 0.004 |
| Na-800-1:1 | 1 | 800 | 0.5 | 37.34 | 35.49 | 1.851 |
| Na-800-1:2 | 1 | 800 | 0.66 | 32.65 | 33.51 | 0.890 |
| Na-800-2:1 | 1 | 800 | 0.33 | 36.36 | 37.32 | 0.959 |
| Na-840-1:1 | 1 | 840 | 0.5 | 35.26 | 35.26 | 0.001 |
| K-760-2:1 | 2 | 760 | 0.33 | 45.59 | 45.58 | 0.013 |
| K-760-1:2 | 2 | 760 | 0.66 | 14.74 | 14.73 | 0.007 |
| K-800-1:1 | 2 | 800 | 0.5 | 37.52 | 37.52 | 0.003 |
| K-840-1:2 | 2 | 840 | 0.66 | 22.70 | 22.70 | 0.003 |
| K-840-2:1 | 2 | 840 | 0.33 | 35.03 | 35.03 | 0.001 |
| N2-760 | 0 | 760 | 0 | 26.33 | 26.33 | 0.000 |
| N2-800 | 0 | 800 | 0 | 11.65 | 11.65 | 0.000 |
| N2-840 | 0 | 840 | 0 | 6.38 | 6.38 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-García, E.A.; Pérez-Huertas, S.; Pérez, A.; Calero, M.; Blázquez, G. Recycling PVC Waste into CO2 Adsorbents: Optimizing Pyrolysis Valorization with Neuro-Fuzzy Models. Processes 2024, 12, 431. https://doi.org/10.3390/pr12030431
Jiménez-García EA, Pérez-Huertas S, Pérez A, Calero M, Blázquez G. Recycling PVC Waste into CO2 Adsorbents: Optimizing Pyrolysis Valorization with Neuro-Fuzzy Models. Processes. 2024; 12(3):431. https://doi.org/10.3390/pr12030431
Chicago/Turabian StyleJiménez-García, Emilia A., Salvador Pérez-Huertas, Antonio Pérez, Mónica Calero, and Gabriel Blázquez. 2024. "Recycling PVC Waste into CO2 Adsorbents: Optimizing Pyrolysis Valorization with Neuro-Fuzzy Models" Processes 12, no. 3: 431. https://doi.org/10.3390/pr12030431
APA StyleJiménez-García, E. A., Pérez-Huertas, S., Pérez, A., Calero, M., & Blázquez, G. (2024). Recycling PVC Waste into CO2 Adsorbents: Optimizing Pyrolysis Valorization with Neuro-Fuzzy Models. Processes, 12(3), 431. https://doi.org/10.3390/pr12030431