Toxicological Activity of Some Plant Essential Oils Against Tribolium castaneum and Culex pipiens Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extraction of Essential Oils
2.2. GC-MS Analysis Conditions
2.3. Red Flour Beetle Rearing
2.4. Mosquito Rearing
2.5. Fumigant Assay on Red Flour Beetle
2.6. Bioassay Toxicity of Mosquitos
2.7. Statistical Analysis
3. Results
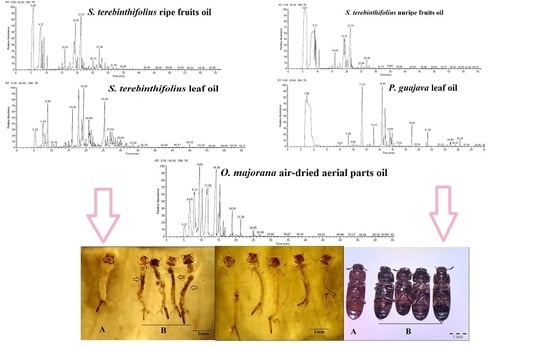
3.1. Chemical Composition of Essential Oils
3.2. Red Flour Beetle Experiment
Fumigant Toxicity of Tested Essential Oils
3.3. Insecticidal Activity of Essential Oil on C. Pipiens
3.3.1. Immature Stages
3.3.2. Adult Stage
3.4. Lethal Concentrations of LC50
4. Discussion
4.1. Chemical Constituents of the Essential Oils
4.2. Fumigant Toxicity on T. Castaneum
4.3. Mosquitocide Activity of Tested Essential Oils
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cavalcanti, E.S.B.; Morais, S.M.; Lima, M.A.A.; Santana, E.W.P. Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Mem. Inst. Oswaldo Cruz 2004, 99, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Huang, C.; Chen, Y.; Yu, J.; Chen, W.; Chang, S. Chemical compositions and larvicidal activities of leaf essential oils from two eucalyptus species. Biores. Technol. 2009, 100, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.; Ahmed, M.H.M.; Yousef, H.; Abdel-Rahman, A.A.H. Repellent and insecticidal activities of Melia azedarach L. against cotton leafworm, Spodoptera littoralis (Boisd.). Z. Naturforsch. 2011, 66, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Rajamma, A.J.; Dubey, S.; Sateesha, S.B.; Tiwari, S.N.; Ghosh, S.K. Comparative larvicidal activity of different species of Ocimum against Culex quinquefasciatus. Nat. Prod. Res. 2011, 25, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chowdhury, N.; Chandra, G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012, 135, 581–598. [Google Scholar] [PubMed]
- Govindarajan, M.; Sivakumar, R.; Rajeswary, M.; Yogalakshmi, K. Chemical composition and larvicidal activity of essential oil from Ocimum basilicum (L.) against Culex tritaeniorhynchus Aedes albopictus and Anopheles subpictus (Diptera: Culicidae). Exp. Parasitol. 2013, 134, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Salem, M.Z.M.; Ashmawy, N.A.; Yessoufou, K.; El-Settawy, A.A. In vitro antibacterial, antifungal, and antioxidant activities of Eucalyptus spp. leaf extracts related to phenolic composition. Nat. Prod. Res. 2017, 31, 2927–2930. [Google Scholar] [CrossRef]
- Hussein, H.S.; Salem, M.Z.M.; Soliman, A.M. Repellent, attractive, and insecticidal effects of essential oils from Schinus terebinthifolius fruits and Corymbia citriodora leaves on two whitefly species, Bemisia tabaci and Trialeurodes ricini. Sci. Horticul. 2017, 216, 111–119. [Google Scholar] [CrossRef]
- Hamada, H.M.; Awad, M.; El-Hefny, M.; Moustafa, M.A.M. Insecticidal activity of garlic (Allium sativum) and ginger (Zingiber officinale) oils on the cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Afr. Entomol. 2018, 26, 84–94. [Google Scholar] [CrossRef]
- Hamad, Y.K.; Abobakr, Y.; Salem, M.Z.M.; Ali, H.M.; Al-Sarar, A.S.; Al-Zabib, A.A. Activity of plant extracts/essential oils against three plant pathogenic fungi and mosquito larvae: GC/MS Analysis of Bioactive Compounds. BioResources 2019, 14, 4489–4511. [Google Scholar]
- Isman, M.B.; Machial, C.; Miresmailli, S.; Bainard, L. Essential oil-based pesticides: New insights from old chemistry. In Pesticide Chemistry; Ohkawa, H., Miyagawa, H., Lee, P., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp. 201–209. [Google Scholar]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Badawy, M.E.I.; El-arami, S.A.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, N.R.; Salem, M.Z.M.; Ali, H.M.; Mackled, M.I.; EL-Hefny, M.; Elshikh, M.S.; Hatamleh, A.A. Morphological, biochemical, molecular, and oil toxicity properties of Taxodium trees from different locations. Ind. Crops Prod. 2019, 139, 111515. [Google Scholar] [CrossRef]
- Wang, C.F.; Yang, K.; You, C.X.; Zhang, W.J.; Guo, S.S.; Geng, Z.F.; Du, S.S.; Wang, Y.Y. Chemical composition and insecticidal activity of essential oils from Zanthoxylum dissitum leaves and roots against Three Species of Storage Pests. Molecules 2015, 20, 7990–7999. [Google Scholar] [CrossRef]
- Wahba, T.F.; Mackled, M.I.; Selim, S.; El-Zemity, S.R. Toxicity and reproduction inhibitory effects of some monoterpenes against the cowpea weevil Callosobruchus maculatus F. (Coleoptera: Chrysomelidae: Bruchinae). Middle East J. Appl. Sci. 2018, 8, 1061–1070. [Google Scholar]
- Russell, T.L.; Kay, B.H.; Skilleter, G.A. Environmental effects of mosquito insecticides on saltmarsh invertebrate fauna. Aquat. Biol. 2009, 6, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Alkenani, N.A. Influence of the mixtures composed of slow–release insecticide formulations against Aedes aegypti mosquito larvae reared in pond water. Saudi J. Biol. Sci. 2017, 24, 1181–1185. [Google Scholar] [CrossRef]
- Murray, B.I. Botanical insecticides, deterrents and repellents in modern and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar]
- Chandler, D.; Bailey, A.S.; Tatchell, G.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Silva, A.G.; Almeida, D.L.; Ronchi, S.N.; Bento, A.C.; Scherer, R.; Ramos, A.C.; Cruz, Z.M.A. The essential oil of Brazilian pepper, Schinus terebinthifolia Raddi in larval control of Stegomyia aegypti (Linnaeus, 1762). Parasites Vectors 2010, 3, 79. [Google Scholar] [CrossRef] [Green Version]
- Ennigrou, A.; Casabianca, H.; Laarif, A.; Hanchi, B.; Hosni, K. Maturation-related changes in phytochemicals and biological activities of the Brazilian pepper tree (Schinus terebinthifolius Raddi) fruits. S. Afr. J. Bot. 2017, 108, 407–415. [Google Scholar] [CrossRef]
- Procópio, T.F.; Fernandes, K.M.; Pontual, E.V.; Ximenes, R.M.; Oliveira, A.R.; Souza, C.S.; Albuquerque Melo, A.M.; Amaral Ferraz Navarro, D.M.; Paiva, P.M.; Martins, G.F.; et al. Schinus terebinthifolius leaf extract causes midgut damage, interfering with survival and development of Aedes aegypti larvae. PLoS ONE 2015, 10, e0126612. [Google Scholar] [CrossRef] [Green Version]
- Kweka, E.J.; Nyindo, M.; Mosha, F.; Silva, A.G. Insecticidal activity of the essential oil from fruits and seeds of Schinus terebinthifolia Raddi against African malaria vectors. Parasites Vectors 2011, 4, 129. [Google Scholar] [CrossRef] [Green Version]
- Mendes, L.A.; Martins, G.F.; Valbon, W.R.; da Silva de Souza, T.; Menini, L.; Ferreira, A.; da Silva Ferreira, M.F. Larvicidal effect of essential oils from Brazilian cultivars of guava on Aedes aegypti L. Ind. Crops Prod. 2017, 108, 684–689. [Google Scholar] [CrossRef]
- Lima, M.A.A.; Oliveira, F.M.M.; Gomes, G.A.; Lavor, P.L.; Santiago, G.M.P.; Nagao-Dias, A.T.; Arriaga, A.M.C.; Lemos, T.L.G.; Carvalho, M.G. Evaluation of larvicidal activity of the essential oils of plants species from Brazil against Aedes aegypti (Diptera: Culicidae). Afr. J. Biotechnol. 2011, 10, 11716–11720. [Google Scholar]
- Satyal, P.; Paudel, P.; Lamichhane, B.; Setzer, W.N. Leaf essential oil composition and bioactivity of Psidium guajava from Kathmandu, Nepal. Amer. J. Essen. Oils Nat. Prod. 2015, 3, 11–14. [Google Scholar]
- El-Akhal, F.; Lalami, A.E.O.; Zoubi, Y.E.; Greche, H.; Guemmouh, R. Chemical composition and larvicidal activity of essential oil of Origanum majorana (Lamiaceae) cultivated in Morocco against Culex pipiens (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2014, 4, 746–750. [Google Scholar] [CrossRef]
- Azizi, A.; Yan, F.; Honermeier, B. Herbage yield, essential oil content and composition of three oregano (Origanum vulgare L.) populations as affected by soil moisture regimes and nitrogen supply. Ind. Crops Prod. 2009, 29, 554–561. [Google Scholar] [CrossRef]
- Pavela, R. Larvicidal effects of various Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol. Res. 2008, 102, 555–559. [Google Scholar] [CrossRef]
- Zahran, H.A.; Abdelgaleil, S.A.M. Insecticidal and developmental inhibitory properties of monoterpenes on Culex pipiens L. (Diptera: Culicidae). J. Asia Pac. Entomol. 2011, 14, 46–51. [Google Scholar]
- WHO. World Malaria Report 2016; WHO Press: Geneva, Switzerland, 2016. [Google Scholar]
- Rajashekar, Y.; Bakthavatsalam, N.; Shivanandappa, T. Botanicals as Grain Protectants. Psyche 2012, 2012, 646740. [Google Scholar] [CrossRef]
- Talukder, F.A. Plant products as potential stored product insect management agents–A mini review. Emir. J. Agric. Sci. 2000, 18, 17–32. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Reichmuth, C. Bioactivity of eugenol, a major component of essential oil of Ocimum suave (Wild.) against four species of stored-product Coleopteran. Inter. J. Pest Manag. 1997, 43, 89–94. [Google Scholar] [CrossRef]
- Asawalam, E.F.; Adesiyan, S.O. Potentials of Ocimum basilicum (Linn.) for the control of Sitophilus zeamais (Motsch). Niger. Agric. J. 2001, 32, 195–201. [Google Scholar]
- Rajashekar, Y.; Gunasekaran, N.; Shivanandappa, T. Insecticidal activity of the root extract of Decalepis hamiltonii against stored-product insect pests and its application in grain protection. J. Food Sci. Technol. 2010, 47, 310–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, K.C.; Devi, S.S. Insecticidal and oviposition deterrent properties of some spices against coleopteran beetle, Sitophilus oryzae. J. Food Sci. Technol. 2011, 50, 600–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, S.; Sriranjini, V. Plant products as fumigants for stored-product insect control. J. Stored Prod. Res. 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Mackled, M.I.; EL-Hefny, M.; Bin-Jumah, M.; Wahba, T.F.; Allam, A.A. Assessment of the toxicity of natural oils from Mentha piperita, Pinus roxburghii, and Rosa spp. against three stored product insects. Processes 2019, 7, 861. [Google Scholar] [CrossRef] [Green Version]
- Okla, M.K.; Alamri, S.A.; Salem, M.Z.M.; Ali, H.M.; Behiry, S.I.; Nasser, R.A.; Alaraidh, I.A.; Al-Ghtani, S.M.; Soufan, W. Yield, phytochemical constituents, and antibacterial activity of essential oils from the leaves/twigs, branches, branch wood, and branch bark of Sour Orange (Citrus aurantium L.). Processes 2019, 7, 363. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.Z.M.; Elansary, H.O.; Ali, H.M.; El-Settawy, A.A.; Elshikh, M.S.; Abdel-Salam, E.M.; Skalicka-Woźniak, K. Antibacterial, antifungal, and antioxidant activities of essential oils extracted from Cupressus macrocarpa branchlets and Corymbia citriodora leaves grown in Egypt. BMC Complement. Alter. Med. 2018, 18, 23. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; EL-Hefny, M.; Ali, H.M.; Elansary, H.O.; Nasser, R.A.; El-Settawy, A.A.A.; El Shanhorey, N.; Ashmawy, N.A.; Salem, A.Z.M. Antibacterial activity of extracted bioactive molecules of Schinus terebinthifolius ripened fruits against some pathogenic bacteria. Microb. Pathogen. 2018, 120, 119–127. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatograph/Quadruple Mass Spectroscopy; Allured Publishing: Carol Stream, IL, USA, 1995; p. 456. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J. Chromatog. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Mansour, M.M.A.; Elansary, H.O. Evaluation of the effect of inner and outer bark extracts of Sugar Maple (Acer saccharum var. saccharum) in combination with citric acid against the growth of three common molds. J. Wood Chem. Technol. 2019, 39, 136–147. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Behiry, S.I.; EL-Hefny, M. Inhibition of Fusarium culmorum, Penicillium chrysogenum and Rhizoctonia solani by n-hexane extracts of three plant species as a wood-treated oil fungicide. J. Appl. Microbiol. 2019, 126, 1683–1699. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Mansour, M.M.A.; Salem, M.Z.M. Lemna gibba and Eichhornia crassipes extracts: Clean alternatives for deacidification, antioxidation and fungicidal treatment of historical paper. J. Clean. Prod. 2019, 219, 846–855. [Google Scholar] [CrossRef]
- EL-Hefny, M.; Ashmawy, N.A.; Salem, M.Z.M.; Salem, A.Z.M. Antibacterial activity of the phytochemicals-characterized extracts of Callistemon viminalis, Eucalyptus camaldulensis and Conyza dioscoridis against the growth of some phytopathogenic bacteria. Microb. Pathogen. 2017, 113, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis, 2nd ed.; Cambridge University Press: Cambridge, London, UK, 1971; p. 318. [Google Scholar]
- El-Bakry, A.M.; Abdel-Aziz, N.F.; Sammour, E.A.; Abdelgaleil, S.A.M. Insecticidal activity of natural plant essential oils against some stored product insects and their side effects on wheat seed germination. Egypt. J. Biol. Pest Cont. 2016, 26, 83–88. [Google Scholar]
- Huang, Y.; Lam, S.L.; Ho, S.H. Bioactivities of essential oil from Elletaria cardamomum (L.) Maton. to Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J. Stored Prod. Res. 2000, 36, 107–117. [Google Scholar] [CrossRef]
- SAS. Users Guide: Statistics (Release 8.02); SAS Inst Inc.: Cary, NC, USA, 2001. [Google Scholar]
- Barbosa, L.C.A.; Demuner, A.J.; Clemente, A.D.; Fonseca de Paula, V.; Ismail, F.M.D. Seasonal variation in the composition of volatile oils from Schinus terebinthifolius Raddi. Quim. Nova 2007, 30, 1959–1965. [Google Scholar] [CrossRef] [Green Version]
- Atti dos Santos, A.C.; Rossato, M.; Agostini, F.; Atti Serafini, L.; Luciana dos Santos, P.; Molon, R.; Dellacassa, E.; Moyna, P. Chemical composition of the essential oils from leaves and fruits of Schinus molle L. and Schinus terebinthifolius Raddi from Southern Brazil. J. Essen. Oil Bear. Plant. 2009, 12, 16–25. [Google Scholar] [CrossRef]
- Cole, E.R.; dos Santos, R.B.; Lacerda Júnior, V.; Martins, J.D.L.; Greco, S.J.; Cunha Neto, A. Chemical composition of essential oil from ripe fruit of Schinus terebinthifolius Raddi and evaluation of its activity against wild strains of hospital origin. Braz. J. Microbiol. 2014, 45, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Périno-Issartier, S.; Abert-Vian, M.; Petitcolas, E.; Chemat, F. Microwave turbo hydrodistillation for rapid extraction of the essential oil from Schinus terebinthifolius Raddi berries. Chromatographia 2010, 72, 347–350. [Google Scholar] [CrossRef]
- Richter, R.; von Reuß, S.H.; König, W.A. Spirocyclopropane-type sesquiterpene hydrocarbons from Schinus terebinthifolius Raddi. Phytochemistry 2010, 71, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus Molle, L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, 466–472. [Google Scholar] [CrossRef] [PubMed]
- El-Shazli, E.M.; Hafezh, S.S.; Abdel-Ghany, A.E. Analysis of the essential oils of S. terebinthifolius Raddi cultivated in Egypt. Zag. J. Pharm. Sci. 2000, 9, 1–8. [Google Scholar]
- Ibrahim, M.T.; Fobbe, R.; Nolte, J. Chemical composition and biological studies of Egyptian Schinus molle L. and Schinus terebinthifolius Raddi oils. Bull. Facul Pharm. Cairo Univ. 2004, 42, 289. [Google Scholar]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Benelli, G. Larvicidal potential of carvacrol and terpinen-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Res. Veter. Sci. 2016, 104, 77–82. [Google Scholar] [CrossRef]
- Traboulsi, A.F.; Taoubi, K.; El-Haj, S.; Bessiere, J.M.; Rammal, S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicideae). Pest Manag. Sci. 2002, 58, 491–495. [Google Scholar] [CrossRef]
- Pino, J.A.; Agüero, J.; Marbot, R.; Fuentes, V. Leaf oil of Psidium guajava L. from Cuba. J. Essen. Oil Res. 2001, 13, 61–62. [Google Scholar] [CrossRef]
- Ji, X.D.; Pu, Q.L.; Garraffo, H.M.; Pannell, L.K. The Essential oil of the leaves of Psidium guajava L. J. Essen. Oil Res. 1991, 3, 187–189. [Google Scholar] [CrossRef]
- Silva, J.A.A.; Hall, D.G.; Gottwald, T.R.; Andrade, M.S.; Maldonado, W., Jr.; Alessandro, R.T.; Lapointe, S.L.; Andrade, E.C.; Machado, M.A. Repellency of selected Psidium guajava cultivars to the Asian citrus psyllid, Diaphorina citri. Crop Protec. 2016, 84, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Weli, A.; Al-Kaabi, A.; Al-Sabahi, J.; Said, S.; Hossain, M.; Al-Riyami, S. Chemical composition and biological activities of the essential oils of Psidium guajava leaf. J. King Saud Univ. Sci. 2018. [Google Scholar] [CrossRef]
- Borah, A.; Pandey, S.K.; Haldar, S.; Lal, M. Chemical composition of leaf essential oil of Psidium guajava L. from North East India. J. Essen. Oil Bear. Plant. 2019, 22, 248–253. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Shawir, M.S.; Abou-Taleb, H.K. Chemical composition, insecticidal and biochemical effects of essential oils of different plant species from Northern Egypt on the rice weevil, Sitophilus oryzae L. J. Pestic. Sci. 2016, 89, 219–229. [Google Scholar] [CrossRef]
- Karabörklü, S.; Ayvaz, A.; Yilmaz, S. Bioactivities of different essential oils against the adults of two stored product insects. Pak. J. Zool. 2010, 42, 679–686. [Google Scholar]
- Salaheddine, S.; Zohra, B.; Cheikh, I.C.; Asma, L. Study of the toxicity of essential oils of Origanum majorana on Tribolium castaneum and Plodia interpunctella (stored product insects). Tunis. J. Med. Plants Nat. Prod. 2013, 9, 29–34. [Google Scholar]
- Rozman, V.; Kalinovic, I.; Korunic, Z. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J. Stored Prod. Res. 2007, 43, 349–355. [Google Scholar] [CrossRef]
- Karan, T.; Simsek, S.; Yildiz, I.; Erenler, R. Chemical composition and insecticidal activity of Origanum syriacum L. essential oil against Sitophilus oryzae and Rhyzopertha dominica. Int. J. Sec. Metab. 2018, 5, 87–93. [Google Scholar]
- Iram, N.; Arshad, M.; Akhter, N. Evaluation of Botanical and Synthetic Insecticide for the Control of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Soc. Entomol. Bras. BioAssay 2013, 8, 3. [Google Scholar]
- Miyazawa, M.; Watanabe, H.; Kameoka, H. Inhibition of acetylcholinesterase activity by monoterpenoids with a P-menthane skeleton. J. Agric. Food Chem. 1997, 45, 677–679. [Google Scholar] [CrossRef]
- Picollo, M.I.; Toloza, A.C.; Mougabure Cueto, G.; Zygadlo, J.; Zerba, E. Anticholinesterase and pediculicidal activities of monoterpenoids. Fitoterapia 2008, 79, 271–278. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Hold, K.M.; Sirisoma, N.S.; Ikeda, T.; Narahashi, T.; Casida, J.E. R-Thujone (the active component of absinthe): γ-Aminobutyric acid type A receptor modulation and metabolic detoxification. Proc. Natl. Acad. Sci. USA 2000, 97, 3826–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelgaleil, S.A.M. Chemical composition, insecticidal and fungicidal activities of essential oils isolated from Mentha microphylla and Lantana camara growing in Egypt. Alex. Sci. Exch. J. 2006, 27, 18–28. [Google Scholar]
- Michaelakis, A.; Mihou, A.P.; Koliopoulos, G.; Couladouros, E.A. Attract-and-kill strategy. Laboratory studies on hatched larvae of Culex pipiens. Pest Manag. Sci. 2007, 63, 954–959. [Google Scholar] [CrossRef]
- Kim, N.J.; Byun, S.G.; Cho, J.E.; Chung, K.; Ahn, Y.J. Larvicidal activity of Kaempferia galangal rhizome phenylpropanoids towards three mosquito species. Pest Manag. Sci. 2008, 64, 857–862. [Google Scholar]
- Radwan, M.A.; El-Zemity, S.R.; Mohamed, S.A.; Sherby, S.M. Larvicidal activity of some essential oils, monoterpenoids and their corresponding N-methyl carbamate derivatives against Culex pipiens (Diptera: Culicidae). Int. J. Trop. Insect Sci. 2008, 28, 61–68. [Google Scholar] [CrossRef]
- Abd El Meguid, A.D.; Mahmoud, S.H.; Baz, M.M. Toxicological activity of four plant oils against Aedes caspius and Culex pipiens (Diptera: Culicidae). Int. J. Mosq. Res. 2019, 6, 86–94. [Google Scholar]
- Sowmyashree, K.; Chalannavar, R.K.; Ghosh, S.K.; Nityasree, B.R.; Supriya, S. Effect of essential oils of Aegle marmelos (L.) correa and Psidium guajava L. on larvae of malaria vector Anopheles stephensi Liston. Res. J. Life Sci Bioinform. Pharm. Chem. Sci. 2019, 5, 705. [Google Scholar] [CrossRef]





| Compound Name | S. terebinthifolius Ripe Fruit Oil | S. terebinthifolius Unripe Fruit Oil | S. terebinthifolius Leaf Oil |
|---|---|---|---|
| α-Pinene | – | 48.9 (696–696) | 4.1 (933–933) |
| Δ-3-Carene | 25.9 (675–689) | – | – |
| β-Pinene | – | – | 0.7 (880–888) |
| Terpinen-4-ol | – | – | 0.2 (869–876) |
| γ-Terpinene | 19.4 (657–709) | 1.8 (818–821) | – |
| α-Thujene | – | 7.7 (670–736) | 0.5 (907–929) |
| D-Limonene | 2.9 (918–919) | 4.3 (838–840) | 2.2 (921–928) |
| Sabinene | – | 4.9 (861–873) | – |
| β-Phellandrene | 1.2 (868–871) | – | 5.9 (833–835) |
| p-Cymene | 4.5 (889–892) | – | 9.1 (890–890) |
| Terpinolene | 1.3 (891–894) | 1.8 (912–913) | 0.4 (892–915) |
| Cymene | – | 2.9 (889–890) | – |
| Linalool | – | – | 0.5 (896–905) |
| α,2-Dimethyl styrene | – | – | 0.4 (897–926) |
| Carvenone | 0.3 (777–788) | 0.1 (780–790) | 0.7 (821–842) |
| α-Terpineol | – | – | 0.5 (903–919) |
| trans-Piperitol | – | – | – |
| cis-Sabinol | 0.6 (911–920) | 0.3 (904–915) | 0.7 (869–890) |
| p-Cymen-8-ol | – | – | 0.2 (863–902) |
| γ-Terpinyl acetate | – | – | – |
| Δ-Elemene | 2.1 (866–873) | 1.8 (871–899) | 0.5 (884–888) |
| Naphthalene | – | – | 5.4 (745–847) |
| γ-Muurolene | 0.45 (734–757) | 0.21 (728–736) | 0.3 (825–837) |
| β-Elemene | – | – | 9.2 (896–899) |
| Citronellyl acetate | 1.1 (807–847) | 0.4 (748–805) | – |
| Aromandendrene | – | – | 3.9 (780–789) |
| Neryl acetate | – | – | – |
| α-Ylangene | 5.3 (853–856) | 2.3 (854–859) | 0.1 (781–787) |
| γ-Elemene | 7.1 (813–848) | 3.7 (830–853) | 11.7 (892–895) |
| IsoGermacrene-D | 0.9 (883–896) | 0.7 (885–893) | 0.6 (911–868) |
| γ-Cadinene | 1.4 (817–837) | 0.9 (805–850) | 0.3 (848–887) |
| γ-Selinene | – | – | 0.3 (877–879) |
| Germacrene D | 14.7 (893–894) | 12.9 (886–889) | 3.4 (908–911) |
| β-Selinene | – | – | 1.7 (912–933) |
| β-Copaene | 1.1 (855–867) | 0.6 (850–864) | 0.2 (868–883) |
| Valencene | 0.3 (831–844) | – | – |
| (+)-Lepidozene | – | 0.2 (830–886) | 2.1 (831–898) |
| Δ-Cadinene | 0.7 (903–908) | 0.2 (869–892) | 1.1 (906–914) |
| Guaiene | – | – | 1.6 (827–828) |
| Selina-3,7(11)-diene | – | – | 0.4 (840–868) |
| Calamenene | – | – | 0.3 (793–897) |
| trans-Sesquisabinene hydrate | – | 0.1 (733–831) | – |
| Globulol | – | – | 0.5 (34–863) |
| Elemoyl acetate | 0.2 (830–870) | 0.1 (797–817) | – |
| α-Costol | – | – | 0.4 (702–756) |
| 4(15),5,10(14)-Germacratrien-1-ol | 0.3 (763–772) | 0.2 (816–822) | 1.4 (804–816) |
| α-Calacorene | – | – | 0.1 (788–923) |
| Spathulenol | 1.2 (872–895) | 0.3 (867–869) | 10.1 (832–852) |
| 4(14)-Salvialen-1-one | 0.2 (800–851) | – | – |
| Rosifoliol | – | – | 0.7 (782–873) |
| β-Neoclovene | – | 0.1 (788–796) | – |
| Eudesma-4,11-dien-2-ol | 0.5 (794–800) | 0.1 (761–767) | 0.1 (774–800) |
| Cubebol | – | – | 0.1 (765–822) |
| Isospathulenol | 2.3 (872–876) | 1.1 (844–849) | 3.5 (865–876) |
| Isoaromadendrene epoxide | – | 0.1 (780–790) | – |
| β-Caryophyllene oxide | 0.3 (777–799) | – | – |
| 11-Hexadecynal | – | – | 1.6 (729–760) |
| α-Cadinol | 0.2 (852–863) | – | 0.3 (821–852) |
| Neointermedeol | – | – | 1.3 (828–861) |
| β-Vetivol | 2.1 (803–814) | 0.1 (810–823) | 0.9 (804–823) |
| α-Costol | – | – | 0.8 (816–847) |
| β-Isonootkatol | 0.2 (813–826) | – | – |
| Aristolene epoxide | – | – | 0.5 (763–787) |
| 8-Hydroxy-endo-Cycloisolongifolene | 0.2 (770–781) | – | – |
| Aromadendrene oxide-(2) | – | – | 1.0 (823–878) |
| cis-9-Hexadecenal | – | – | 0.1 (721–745) |
| (Z)-9,17-Octadecadienal | – | – | 0.1 (713–805) |
| Anthracene | – | – | 0.2 (908–953) |
| Viridiflorene | – | – | 0.5 (869–870) |
| Compound Name | Relative Quantity (%) | Standard Index | Reverse Standard Index |
|---|---|---|---|
| α-Pinene | 25.5 | 834 | 836 |
| Δ-3-Carene | 8.8 | 787 | 788 |
| β-Pinene | 0.5 | 902 | 912 |
| Camphene | 0.2 | 871 | 875 |
| trans-Isolimonene | 0.2 | 864 | 865 |
| Terpinen-4-ol | 0.3 | 908 | 910 |
| β-Fenchol | 0.5 | 830 | 849 |
| L-Bornyl acetate | 2.2 | 929 | 929 |
| trans-Pinocarvyl acetate | 0.2 | 818 | 851 |
| Bornylene | 0.5 | 820 | 829 |
| Bicycloelemene | 0.2 | 776 | 847 |
| α-Patchoulene | 0.3 | 778 | 796 |
| Cedrene | 0.11 | 880 | 898 |
| β-Chamigrene | 0.2 | 895 | 913 |
| β-Himachalene | 0.3 | 901 | 910 |
| Thujopsene-(I2) | 0.1 | 890 | 900 |
| Cuparene | 2.6 | 892 | 894 |
| (E)-Caryophyllene | 15.7 | 872 | 874 |
| γ-Muurolene | 0.2 | 881 | 896 |
| (E)-Nerolidol | 16.7 | 870 | 871 |
| Aristolene epoxide | 0.7 | 757 | 803 |
| Cedran-8-ol | 8.8 | 878 | 882 |
| Widdrol | 0.6 | 747 | 750 |
| Isospathulenol | 0.2 | 815 | 861 |
| α-Bisabolol | 2.1 | 834 | 885 |
| Ledene oxide-(II) | 1.1 | 835 | 837 |
| 1,3,3-Trimethyl-2-(2-methyl-cyclopropyl)-cyclohexene | 1.4 | 758 | 805 |
| 8-Hydroxy-endo-Cycloisolongifolene | 0.3 | 812 | 836 |
| Calarene epoxide | 0.1 | 753 | 814 |
| Viridiflorene | 0.1 | 771 | 781 |
| Labda-8(20),12,14-triene | 0.1 | 791 | 794 |
| 13-Epimanool | 3.3 | 778 | 785 |
| Compound Name | Relative Quantity (%) | Standard Index | Reverse Standard Index |
|---|---|---|---|
| γ-Terpinene | 16.5 | 877 | 880 |
| α-Thujene | 3.2 | 915 | 922 |
| Sabinene | 10.1 | 915 | 924 |
| α-Terpinene | 6.1 | 895 | 897 |
| β-Thujene | 0.9 | 910 | 931 |
| β-Phellandrene | 0.7 | 910 | 915 |
| p-Cymene | 0.8 | 839 | 875 |
| Terpinolene | 5.7 | 898 | 900 |
| γ-Terpineol | 5.1 | 845 | 847 |
| 4-Thujanol | 2.6 | 899 | 902 |
| trans-4-Thujanol | 4.8 | 916 | 918 |
| cis-Para-2-menthen-1-ol | 0.3 | 884 | 886 |
| (E)-Caryophyllene | 2.5 | 894 | 895 |
| Terpinen-4-ol | 21.7 | 876 | 882 |
| α-Terpineol | 5.1 | 914 | 925 |
| trans-Piperitol | 0.1 | 833 | 865 |
| γ-Terpinyl acetate | 6.7 | 830 | 830 |
| Bornyl acetate | 0.3 | 911 | 945 |
| Terpinyl propionate | 0.6 | 845 | 886 |
| Neryl acetate | 0.5 | 891 | 897 |
| γ-Elemene | 1.4 | 886 | 906 |
| α-Humulene | 0.1 | 863 | 880 |
| Germacrene D | 1.6 | 892 | 894 |
| Spathulenol | 0.5 | 902 | 903 |
| Isospathulenol | 0.2 | 828 | 843 |
| β-Caryophyllene oxide | 0.2 | 858 | 867 |
| Source of Essential Oil | Concentration (µg/L Air) | Time (h) | ||||
|---|---|---|---|---|---|---|
| 6 | 12 | 24 | 48 | 72 | ||
| S. terebinthifolius ripe fruits | 0 | 0 | 1.6 ± 2.8 | 1.66 ± 2.88 | 3.3 ± 2.8 | 8.3 ± 2.8 |
| 2 | 0 | 16.6 ± 14.4 | 33.33 ± 14.43 | 58.3 ± 7.6 | 83.3 ± 7.6 | |
| 5 | 6.6 ± 5.7 | 30 ± 5 | 58.33 ± 7.63 | 76.6 ± 10.4 | 86.6 ± 5.7 | |
| 10 | 6.6 ± 5.7 | 33.3 ± 2.8 | 70.00 ± 0.00 | 85 ± 5 | 100 | |
| 20 | 31.6 ± 2.8 | 50 ± 5 | 83.3 ± 7.6 | 100 | 100 | |
| 40 | 61.6 ± 12.5 | 80 ± 10 | 100 | 100 | 100 | |
| S. terebinthifolius unripe fruits | 0 | 0 | 1.6 ± 2.8 | 1.6 ± 2.8 | 3.3 ± 2.8 | 8.33 ± 2.88 |
| 2 | 35 ± 5 | 51.6 ± 7.6 | 78.3 ± 7.6 | 100 | 100 | |
| 5 | 43.3 ± 11.5 | 75 ± 5 | 100 | 100 | 100 | |
| 10 | 55 ± 18 | 100 | 100 | 100 | 100 | |
| 20 | 61.6 ± 12.5 | 100 | 100 | 100 | 100 | |
| 40 | 76.6 ± 5.7 | 100 | 100 | 100 | 100 | |
| S. terebinthifolius leaves | 0 | 0 | 1.6 ± 2.8 | 1.6 ± 2.8 | 3.3 ± 2.8 | 8.3 ± 2.8 |
| 2 | 0 | 8.3 ± 2.8 | 28.3 ± 5.7 | 55 ± 5 | 75 ± 10 | |
| 5 | 0 | 26.6 ± 15.2 | 50 ± 5 | 73.3 ± 2.8 | 90 ± 5 | |
| 10 | 8.3 ± 7.6 | 33.3 ± 7.6 | 70 ± 8.6 | 86.6 ± 5.7 | 100 | |
| 20 | 10 | 68.3 ± 17.5 | 85 ± 10 | 100 | 100 | |
| 40 | 40 ± 10 | 76.6 ± 5.7 | 100 | 100 | 100 | |
| O. majorana leaves | 0 | 0 | 1.66 ± 2.8 | 1.6 ± 2.8 | 3.3 ± 2.8 | 8.3 ± 2.8 |
| 2 | 0 | 0 | 6.6 ± 2.8 | 16.6 ± 2.8 | 26.6 ± 2.8 | |
| 5 | 0 | 0 | 10 | 21.6 ± 2.8 | 28.3 ± 2.8 | |
| 10 | 0 | 0 | 11.6 ± 5.7 | 26.6 ± 2.8 | 31.6 ± 5.7 | |
| 20 | 0 | 5 ± 5 | 20 ± 5 | 33.3 ± 5.7 | 45 ± 5 | |
| 40 | 0 | 8.3 ± 2.8 | 31.6 ± 7.6 | 56.6 ± 15.2 | 68.3 ± 12.5 | |
| P. guajava leaves | 0 | 0 | 1.6 ± 2.8 | 1.6 ± 2.8 | 3.3 ± 2.8 | 8.3 ± 2.8 |
| 2 | 0 | 18.3 ± 7.6 | 41.6 ± 7.6 | 61.6 ± 5.7 | 83.3 ± 12.5 | |
| 5 | 5 ± 5 | 30 ± 5 | 55 ± 5 | 70 ± 5 | 88.3 ± 12.5 | |
| 10 | 28.3 ± 7.6 | 60 ± 10 | 73.3 ± 7.6 | 86.6 ± 5.7 | 91.6 ± 14.4 | |
| 20 | 26.6 ± 2.8 | 63.3 ± 12.5 | 73.3 ± 7.6 | 86.6 ± 5.7 | 100 | |
| 40 | 36.6 ± 5.7 | 65 ± 13.2 | 75 ± 13.2 | 93.3 ± 11.5 | 100 | |
| LSD*0.05 = 10.077 | ||||||
| Tested Essential Oil | Period (h) | LC50 (µg/L Air) | 95% Confidence Limits | Slope ± SE* | Chi2 | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| S. terebinthifolius ripe fruits | 6 | 33.3 | 20.3 | 54.6 | 2.1 ± 0.1 | 0.59 |
| 12 | 15.5 | 8 | 30.2 | 1.2 ± 0.1 | 0.92 | |
| 24 | 4.2 | 2.2 | 8.1 | 1.3 ± 0.1 | 0.98 | |
| 48 | <2 | |||||
| 72 | <2 | |||||
| S. terebinthifolius unripe fruits | 6 | 6.8 | 2.5 | 18.4 | 0.8 ± 0.2 | 0.99 |
| 12 | 2 | 1.1 | 3.8 | 1.5 ± 0.1 | NA | |
| 24 | <2 | |||||
| 48 | <2 | |||||
| 72 | <2 | |||||
| S. terebinthifolius leaves | 6 | >40 | ||||
| 12 | 14.5 | 8.5 | 24.5 | 1.7 ± 0.1 | 0.95 | |
| 24 | 5.1 | 2.8 | 8.8 | 1.5 ± 0.1 | 0.99 | |
| 48 | <2 | |||||
| 72 | <2 | |||||
| O. majorana leaves | 6 | >40 | ||||
| 12 | >40 | |||||
| 24 | >40 | |||||
| 48 | >40 | |||||
| 72 | 37.9 | 13.9 | 103.1 | 0.8 ± 0.2 | 0.85 | |
| P. guajava leaves | 6 | >40 | ||||
| 12 | 9.5 | 4.5 | 16.4 | 1.4 ± 0.3 | 0.93 | |
| 24 | 6.1 | 1.8 | 19.7 | 0.6 ± 0.2 | 0.97 | |
| 48 | <2 | |||||
| 72 | <2 | |||||
| S.O.V.* | DF | Sum of Squares | Mean Square | F-test Value | Pr > F |
|---|---|---|---|---|---|
| Larval stage | Mortality after 24 h (%) | ||||
| Oil concentration (A) | 4 | 6235.73 | 1558.93 | 186.18 | <0.0001 |
| Oil source (B) | 4 | 1504 | 376 | 44.90 | <0.0001 |
| A × B | 16 | 988.26 | 61.76 | 7.38 | <0.0001 |
| Error | 50 | 418.66 | 8.37 | ||
| Mortality after 48 h (%) | |||||
| A | 4 | 7212.58 | 1803.146 | 193.19 | <0.0001 |
| B | 4 | 1546.98 | 386.74 | 41.44 | <0.0001 |
| A × B | 16 | 959.14 | 59.94 | 6.42 | <0.0001 |
| Error | 50 | 466.66 | 9.33 | ||
| Total mortality (%) | |||||
| A | 4 | 22,063.14 | 5515.78 | 415.35 | <0.0001 |
| B | 4 | 7092.48 | 1773.12 | 133.52 | <0.0001 |
| A × B | 16 | 2720.85 | 170.053 | 12.81 | <0.0001 |
| Error | 50 | 664 | 13.28 | ||
| Longevity (days) | |||||
| A | 4 | 992.89 | 48.22 | 30.94 | <0.0001 |
| B | 4 | 167.718 | 41.929 | 5.23 | 0.0013 |
| A × B | 16 | 78.8658 | 4.9291 | 0.61 | 0.8571 |
| Error | 50 | 401.153 | 8.023 | ||
| Pupal stage | Mortality (%) | ||||
| A | 4 | 26,178.66 | 6544.66 | 475.63 | <0.0001 |
| B | 4 | 7129.06 | 1782.26 | 129.53 | <0.0001 |
| A × B | 16 | 2540.26 | 158.76 | 11.54 | <0.0001 |
| Error | 50 | 688. | 13.76 | ||
| Longevity (h) | |||||
| A | 4 | 2292.65 | 573.16 | 17.31 | <0.0001 |
| B | 4 | 2734.14 | 683.53 | 20.65 | <0.0001 |
| A × B | 16 | 1040.83 | 65.05 | 1.96 | 0.0355 |
| Error | 50 | 1655.27 | 33.11 | ||
| Adult stage | Mortality (%) | ||||
| A | 4 | 47,423.78 | 11,855.94 | 835.71 | <0.0001 |
| B | 4 | 9540.05 | 2385.01 | 168.12 | <0.0001 |
| A × B | 16 | 2717.54 | 169.84 | 11.97 | <0.0001 |
| Error | 50 | 709.33 | 14.18 | ||
| Longevity (days) | |||||
| A | 4 | 6651.02 | 1662.75 | 94.69 | <0.0001 |
| B | 4 | 1961.75 | 490.43 | 27.93 | <0.0001 |
| A × B | 16 | 767.807 | 47.98 | 2.73 | 0.0034 |
| Error | 50 | 878.04 | 17.56 | ||
| Tested Essential Oil | Concentration (mg/L) | Larval Stage | Pupal Stage | Adult Stage | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mortality after 24 h (%) | Mortality after 48 h (%) | Total Mortality (%) | Longevity (days) | Mortality (%) | Longevity (h) | Mortality (%) | Longevity (days) | ||
| S. terebinthifolius ripe fruits | 0 | 0.6 ± 1.1 | 0.6 ± 1.1 | 3.3 ± 1.1 | 8.3 ± 0.6 | 4 ± 2 | 32.2 ± 6.7 | 5.3 ± 1.1 | 44.3 ± 6.1 |
| 10 | 10 ± 2 | 10 ± 2 | 26 ± 2 | 11.2 ± 0.8 | 26 ± 2 | 36.7 ± 10.5 | 36 ± 2 | 44.6 ± 2.1 | |
| 25 | 10.6 ± 3 | 11.3 ± 2.3 | 26.6 ± 3 | 14.4 ± 2.9 | 26.6 ± 3 | 46.1 ± 12.4 | 38 ± 5.2 | 40.6 ± 3 | |
| 50 | 12 ± 2 | 13.3 ± 2.3 | 30.6 ± 3 | 18.5 ± 3.4 | 32.6 ± 3 | 57 ± 4.4 | 49.3 ± 3 | 32 ± 6.4 | |
| 100 | 15.3 ± 1.1 | 17.3 ± 1.1 | 33.3 ± 3 | 20.6 ± 2.1 | 40 ± 4 | 63.4 ± 1.6 | 58 ± 4 | 27.3 ± 3.2 | |
| S. terebinthifolius unripe fruits | 0 | 0.6 ± 1.1 | 0.6 ± 1.1 | 3.3 ± 1.1 | 8.3 ± 0.6 | 4 ± 2 | 32.2 ± 6.7 | 5.3 ± 1.1 | 44.3 ± 6.1 |
| 10 | 13.3 ± 4.1 | 14 ± 4 | 40.6 ± 9.4 | 9.4 ± 1.8 | 42.6 ± 9.4 | 25.1 ± 6.5 | 58.6 ± 9.4 | 33.7 ± 4.7 | |
| 25 | 18 ± 2 | 18.6 ± 1.1 | 48 ± 2 | 10.5 ± 2.4 | 45.3 ± 5.7 | 29.8 ± 4.7 | 63.3 ± 5.7 | 22.6 ± 4 | |
| 50 | 28 ± 2 | 29.3 ± 2.3 | 60 ± 2 | 13.7 ± 3.2 | 64 ± 2 | 34.4 ± 2.5 | 84 ± 2 | 18.1 ± 1.6 | |
| 100 | 34.6 ± 5.7 | 36.6 ± 5.7 | 68 ± 3.4 | 16.3 ± 0.9 | 72 ± 3.4 | 37.1 ± 6.3 | 94 ± 6 | 15.4 ± 4.4 | |
| S. terebinthifolius leaves | 0 | 0.6 ± 1.1 | 0.6 ± 1.1 | 3.3 ± 1.1 | 8.3 ± 0.6 | 4 ± 2 | 32.2 ± 6.7 | 5.3 ± 1.1 | 44.3 ± 6.1 |
| 10 | 9.3 ± 1.1 | 10 ± 2 | 30 ± 2 | 8.5 ± 5.1 | 30 ± 2 | 33.3 ± 3.9 | 46 ± 2 | 34.4 ± 7.3 | |
| 25 | 15.3 ± 3 | 16 ± 2 | 36 ± 2 | 8.9 ± 4.4 | 38 ± 2 | 34.5 ± 0.7 | 54 ± 2 | 23.6 ± 3.1 | |
| 50 | 27.3 ± 4.1 | 28.6 ± 5 | 48.6 ± 5 | 14.7 ± 5.5 | 52.6 ± 5 | 38.5 ± 4.9 | 70.6 ± 5 | 18.8 ± 2.2 | |
| 100 | 30.6 ± 5 | 32.6 ± 5 | 50 ± 2 | 18.2 ± 2.4 | 58 ± 2 | 43.1 ± 6.4 | 78 ± 2 | 20.5 ± 3.2 | |
| O. majorana leaves | 0 | 0.6 ± 1.1 | 0.6 ± 1.1 | 3.3 ± 1.1 | 8.3 ± 0.6 | 4 ± 2 | 32.2 ± 6.7 | 5.3 ± 1.1 | 44.3 ± 6.1 |
| 10 | 12 ± 5.2 | 12.6 ± 6.4 | 42.6 ± 6.4 | 8.1 ± 2.7 | 44.6 ± 6.4 | 22.1 ± 4.1 | 62.6 ± 6.4 | 30.4 ± 2 | |
| 25 | 19.3 ± 1.1 | 20 ± 2 | 50 ± 2 | 9.3 ± 2.4 | 52 ± 2 | 28.8 ± 3 | 70 ± 2 | 19.4 ± 1.2 | |
| 50 | 30.6 ± 3 | 32 ± 4 | 62 ± 4 | 12.3 ± 4.9 | 66 ± 4 | 30.7 ± 5.1 | 86 ± 4 | 14.8 ± 1.4 | |
| 100 | 36.6 ± 4.1 | 38.6 ± 4.1 | 78 ± 2 | 14.8 ± 2.8 | 82 ± 2 | 34.7 ± 3.7 | 100 | 11.8 ± 2.4 | |
| Psidium guajava leaves | 0 | 0.6 ± 1.1 | 0.6 ± 1.1 | 3.3 ± 1.1 | 8.3 ± 0.6 | 4 ± 2 | 32.2 ± 6.7 | 5.3 ± 1.1 | 44.3 ± 6.1 |
| 10 | 8 ± 2 | 8 ± 2 | 24 ± 2 | 9.5 ± 1.5 | 24 ± 2 | 31.6 ± 3.4 | 34.6 ± 2.3 | 45 ± 3 | |
| 25 | 9.3 ± 1.1 | 10 | 26 | 13.7 ± 3.1 | 27.3 ± 2.3 | 35.2 ± 3.6 | 37.3 ± 2.3 | 38.6 ± 1.1 | |
| 50 | 12.6 ± 3 | 14 ± 3.4 | 30 ± 3.4 | 17.4 ± 1.9 | 33.3 ± 3 | 41.7 ± 3.4 | 51.3 ± 3 | 21.7 ± 4.3 | |
| 100 | 16.6 ± 1.1 | 18.6 ± 1.1 | 36.6 ± 8.1 | 19.9 ± 2.6 | 42.6 ± 5 | 46.4 ± 3.6 | 63.3 ± 3 | 24.9 ± 0.8 | |
| P-value | 0.0002 | <0.0001 | <0.0001 | 0.8571 | <0.0001 | 0.0355 | <0.0001 | 0.0034 | |
| Oil Source | Insect Mortality | LC50 (mg/L) | 95% Confidence Limits | Slope ± SE | Chi2 | R2 | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| S. terebinthifolius ripe fruits | TL* | >100 | – | – | – | – | – |
| Ad* | >50 | – | – | – | – | – | |
| S. terebinthifolius unripe fruits | TL | 31.2 | 8.8 | 109.6 | 0.721 ± 0.2 | 0.9 | 0.9 |
| Ad | 10.9 | 4.7 | 24.9 | 1.185 ± 0.2 | 0.8 | 0.9 | |
| S. terebinthifolius leaf | TL | >100 | – | – | – | – | – |
| Ad | 20.1 | 6.9 | 57.3 | 0.872 ± 0.2 | 0.9 | 0.9 | |
| O. majorana leaf | TL | 24.1 | 8.9 | 64.7 | 0.925 ± 0.2 | 0.9 | 0.9 |
| Ad | 9.7 | 4.6 | 20.1 | 1.414 ± 0.1 | 0.8 | 0.9 | |
| P. guajava leaf | TL | >100 | – | – | – | – | – |
| Ad | >50 | – | – | – | – | – | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sabrout, A.M.; Salem, M.Z.M.; Bin-Jumah, M.; Allam, A.A. Toxicological Activity of Some Plant Essential Oils Against Tribolium castaneum and Culex pipiens Larvae. Processes 2019, 7, 933. https://doi.org/10.3390/pr7120933
El-Sabrout AM, Salem MZM, Bin-Jumah M, Allam AA. Toxicological Activity of Some Plant Essential Oils Against Tribolium castaneum and Culex pipiens Larvae. Processes. 2019; 7(12):933. https://doi.org/10.3390/pr7120933
Chicago/Turabian StyleEl-Sabrout, Ahmed M., Mohamed Z. M. Salem, May Bin-Jumah, and Ahmed A. Allam. 2019. "Toxicological Activity of Some Plant Essential Oils Against Tribolium castaneum and Culex pipiens Larvae" Processes 7, no. 12: 933. https://doi.org/10.3390/pr7120933
APA StyleEl-Sabrout, A. M., Salem, M. Z. M., Bin-Jumah, M., & Allam, A. A. (2019). Toxicological Activity of Some Plant Essential Oils Against Tribolium castaneum and Culex pipiens Larvae. Processes, 7(12), 933. https://doi.org/10.3390/pr7120933