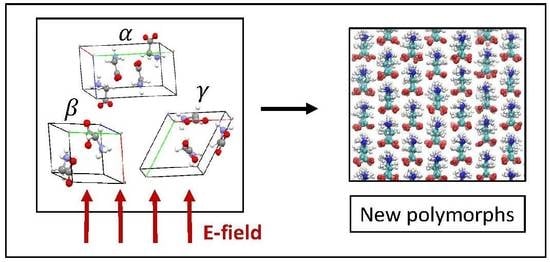
Exploring New Crystal Structures of Glycine via Electric Field-Induced Structural Transformations with Molecular Dynamics Simulations
Abstract
:1. Introduction
2. Methods
2.1. Simulation Details
2.2. Analysis Methods
2.2.1. Dipole Alignment Analysis
2.2.2. Crystal Structure Analysis
3. Results and Discussion
3.1. Effect of Initial Crystal Structure
3.2. Effect of e-Field Strength and Direction
3.3. Analysis of the New Crystals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beckmann, W. Seeding the desired polymorph: Background, possibilities, limitations, and case studies. Org. Process. Res. Dev. 2000, 4, 372–383. [Google Scholar] [CrossRef]
- Cimarosti, Z.; Castagnoli, C.; Rossetti, M.; Scarati, M.; Day, C.; Johnson, B.; Westerduin, P. Development of drug substances as mixture of polymorphs: Studies to control form 3 in casopitant mesylate. Org. Process. Res. Dev. 2010, 14, 1337–1346. [Google Scholar] [CrossRef]
- Weissbuch, I.; Torbeev, V.Y.; Leiserowitz, L.; Lahav, M. Solvent effect on crystal polymorphism: Why addition of methanol or ethanol to aqueous solutions induces the precipitation of the least stable β form of glycine. Angew. Chem. 2005, 117, 3290–3293. [Google Scholar] [CrossRef]
- Kitamura, M.; Hara, T.; Takimoto-Kamimura, M. Solvent effect on polymorphism in crystallization of BPT propyl ester. Cryst. Growth Des. 2006, 6, 1945–1950. [Google Scholar] [CrossRef]
- Adrjanowicz, K.; Paluch, M.; Richert, R. Formation of new polymorphs and control of crystallization in molecular glass-formers by electric field. Phys. Chem. Chem. Phys. 2018, 20, 925–931. [Google Scholar] [CrossRef]
- Potticary, J.; Terry, L.R.; Bell, C.; Papanikolopoulos, A.N.; Christianen, P.C.; Engelkamp, H.; Collins, A.M.; Fontanesi, C.; Kociok-Köhn, G.; Crampin, S.; et al. An unforeseen polymorph of coronene by the application of magnetic fields during crystal growth. Nat. Commun. 2016, 7, 11555. [Google Scholar] [CrossRef] [Green Version]
- Parks, C.; Koswara, A.; Tung, H.H.; Nere, N.; Bordawekar, S.; Nagy, Z.K.; Ramkrishna, D. Molecular dynamics electric field crystallization simulations of paracetamol produce a new polymorph. Cryst. Growth Des. 2017, 17, 3751–3765. [Google Scholar] [CrossRef]
- Svishchev, I.M.; Kusalik, P.G. Crystallization of liquid water in a molecular dynamics simulation. Phys. Rev. Lett. 1994, 73, 975. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Patey, G. Molecular dynamics simulations of ice nucleation by electric fields. J. Phys. Chem. 2012, 116, 7057–7064. [Google Scholar] [CrossRef]
- Svishchev, I.M.; Kusalik, P.G. Quartzlike polymorph of ice. Phys. Rev. B 1996, 53, R8815. [Google Scholar] [CrossRef]
- Garetz, B.; Aber, J.; Goddard, N.; Young, R.; Myerson, A. Nonphotochemical, polarization-dependent, laser-induced nucleation in supersaturated aqueous urea solutions. Phys. Rev. Lett. 1996, 77, 3475. [Google Scholar] [CrossRef]
- Sun, X.; Garetz, B.A.; Myerson, A.S. Supersaturation and polarization dependence of polymorph control in the nonphotochemical laser-induced nucleation (NPLIN) of aqueous glycine solutions. Cryst. Growth Des. 2006, 6, 684–689. [Google Scholar] [CrossRef]
- Aber, J.E.; Arnold, S.; Garetz, B.A.; Myerson, A.S. Strong dc electric field applied to supersaturated aqueous glycine solution induces nucleation of the γ polymorph. Phys. Rev. Lett. 2005, 94, 145503. [Google Scholar] [CrossRef]
- Lee, I.S.; Evans, J.M.; Erdemir, D.; Lee, A.Y.; Garetz, B.A.; Myerson, A.S. Nonphotochemical laser induced nucleation of hen egg white lysozyme crystals. Cryst. Growth Des. 2008, 8, 4255–4261. [Google Scholar] [CrossRef]
- Sun, X.; Garetz, B.A.; Myerson, A.S. Polarization switching of crystal structure in the nonphotochemical laser-induced nucleation of supersaturated aqueous l-histidine. Cryst. Growth Des. 2008, 8, 1720–1722. [Google Scholar] [CrossRef]
- Zaccaro, J.; Matic, J.; Myerson, A.S.; Garetz, B.A. Nonphotochemical, laser-induced nucleation of supersaturated aqueous glycine produces unexpected γ-polymorph. Cryst. Growth Des. 2001, 1, 5–8. [Google Scholar] [CrossRef]
- Parks, C.; Koswara, A.; Tung, H.H.; Nere, N.; Bordawekar, S.; Nagy, Z.K.; Ramkrishna, D. Extending the Crystal Landscape Through Electric Field Controlled Crystallization—A Molecular Dynamics Case Study. ChemRxiv 2018. [Google Scholar] [CrossRef]
- Byrn, S.R.; Zografi, G.; Chen, X.S. Solid-State Properties Of Pharmaceutical Materials; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Yu, L.; Stephenson, G.A.; Mitchell, C.A.; Bunnell, C.A.; Snorek, S.V.; Bowyer, J.J.; Borchardt, T.B.; Stowell, J.G.; Byrn, S.R. Thermochemistry and conformational polymorphism of a hexamorphic crystal system. J. Am. Chem. Soc. 2000, 122, 585–591. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.; Zhang, T.; Zhang, S.; Zhang, J.; Yang, L. First-principles study of electric field effects on the structure, decomposition mechanism, and stability of crystalline lead styphnate. J. Mol. Model. 2014, 20, 2072. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.; Zhang, T.; Xu, J.; Zhang, J.; Yang, L. Electric-Field-Induced Structural and Electronic Changes and Decomposition of Crystalline Lead Azide: A Computational Study. J. Phys. Chem. C 2015, 119, 8431–8437. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [Green Version]
- Cheong, D.W.; Boon, Y.D. Comparative study of force fields for molecular dynamics simulations of α-glycine crystal growth from solution. Cryst. Growth Des. 2010, 10, 5146–5158. [Google Scholar] [CrossRef]
- Jönsson, P.G.; Kvick, Å. Precision neutron diffraction structure determination of protein and nucleic acid components. III. The crystal and molecular structure of the amino acid α-glycine. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 1827–1833. [Google Scholar] [CrossRef]
- Drebushchak, T.N.; Boldyreva, E.V.; Shutova, E.S. β-Glycine. Acta Crystallogr. Sect. Struct. Rep. Online 2002, 58, o634–o636. [Google Scholar] [CrossRef]
- Iitaka, Y. The crystal structure of β-glycine. Acta Crystallogr. 1960, 13, 35–45. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.; Postma, J.V.; van Gunsteren, W.F.; DiNola, A.; Haak, J. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Abraham, M.; Hess, B.; van der Spoel, D.; Lindahl, E. User Manual; Version 5.0.7; The GROMACS Development Teams at the Royal Institute of Technology and Uppsala University: Uppsala, Sweden, 2015. [Google Scholar]
- Mandal, T.; Larson, R.G. Nucleation of urea from aqueous solution: Structure, critical size, and rate. J. Chem. Phys. 2017, 146, 134501. [Google Scholar] [CrossRef]
- Iitaka, Y. The crystal structure of γ-glycine. Acta Crystallogr. 1958, 11, 225–226. [Google Scholar] [CrossRef]
- Di Profio, G.; Reijonen, M.T.; Caliandro, R.; Guagliardi, A.; Curcio, E.; Drioli, E. Insights into the polymorphism of glycine: Membrane crystallization in an electric field. Phys. Chem. Chem. Phys. 2013, 15, 9271–9280. [Google Scholar] [CrossRef]
- Dawson, A.; Allan, D.R.; Belmonte, S.A.; Clark, S.J.; David, W.I.; McGregor, P.A.; Parsons, S.; Pulham, C.R.; Sawyer, L. Effect of high pressure on the crystal structures of polymorphs of glycine. Cryst. Growth Des. 2005, 5, 1415–1427. [Google Scholar] [CrossRef]









| Parameter | Value |
|---|---|
| Initial Structure | -(2000), -(1980) and -(1920) |
| Direction of the electric field | x, y, z |
| Strength of the electric field (V/nm) | 0.25, 0.50, 0.75, 1.00, 1.25, 1.50 |
| Parameter | Potential Energy (kJ/mol) | Enthalpy (kJ/mol) | Cp (J/mol) | Density (kg/m3) | Specific Volume () (m3/mol) |
|---|---|---|---|---|---|
| -gly | 4.67 | ||||
| -gly | 4.68 | ||||
| -gly | 4.68 | ||||
| -y1.5 | 4.61 | ||||
| -y1.5 | 4.92 | ||||
| -y2.0 | 4.63 | ||||
| -y1.5 | 4.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulutoglu, P.S.; Parks, C.; Nere, N.K.; Bordawekar, S.; Ramkrishna, D. Exploring New Crystal Structures of Glycine via Electric Field-Induced Structural Transformations with Molecular Dynamics Simulations. Processes 2019, 7, 268. https://doi.org/10.3390/pr7050268
Bulutoglu PS, Parks C, Nere NK, Bordawekar S, Ramkrishna D. Exploring New Crystal Structures of Glycine via Electric Field-Induced Structural Transformations with Molecular Dynamics Simulations. Processes. 2019; 7(5):268. https://doi.org/10.3390/pr7050268
Chicago/Turabian StyleBulutoglu, Pelin Su, Conor Parks, Nandkishor K. Nere, Shailendra Bordawekar, and Doraiswami Ramkrishna. 2019. "Exploring New Crystal Structures of Glycine via Electric Field-Induced Structural Transformations with Molecular Dynamics Simulations" Processes 7, no. 5: 268. https://doi.org/10.3390/pr7050268
APA StyleBulutoglu, P. S., Parks, C., Nere, N. K., Bordawekar, S., & Ramkrishna, D. (2019). Exploring New Crystal Structures of Glycine via Electric Field-Induced Structural Transformations with Molecular Dynamics Simulations. Processes, 7(5), 268. https://doi.org/10.3390/pr7050268