The Influence of Nitrogen Absorption on Microstructure, Properties and Cytotoxicity Assessment of 316L Stainless Steel Alloy Reinforced with Boron and Niobium
Abstract
:1. Introduction
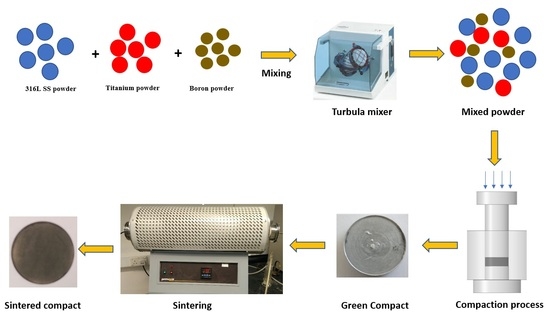
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, C.-W.; Fang, F.-Z. State of the art of bioimplants manufacturing: Part I. Adv. Manuf. 2018, 6, 20–40. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention-a review. Recent Patents Corros. Sci. 2010. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Kang, C.-W.; Fang, F.-Z. State of the art of bioimplants manufacturing: Part II. Adv. Manuf. 2018, 6, 137–154. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Patil, S.; Ramteke, P.W.; Laux, P.; Singh, A.V. In Vivo Biocompatibility of Electrospun Biodegradable Dual Carrier (Antibiotic+ Growth Factor) in a Mouse Model—Implications for Rapid Wound Healing. Pharmaceutics 2019, 11, 180. [Google Scholar] [CrossRef]
- Wiles, P. The surgery of the osteo-arthritic hip. Br. J. Surg. 1958, 45, 488–497. [Google Scholar] [CrossRef]
- Wiles, P. The classic: The surgery of the osteo-arthritic hip. Clin. Orthop. Relat. Res. 2003, 417, 3–16. [Google Scholar]
- Lo, K.H.; Shek, C.H.; Lai, J. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.M.A.; Altaf, K.; Baig, Z. Investigation of Boron addition and compaction pressure on the compactibility, densification and microhardness of 316L Stainless Steel. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Park, J.B. Biomaterials Science and Engineering; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Khuenkaew, T.; Kanlayasiri, K. Resistance Spot Welding of SUS316L Austenitic/SUS425 Ferritic Stainless Steels: Weldment Characteristics, Mechanical Properties, Phase Transformation and Solidification. Metals 2019, 9, 710. [Google Scholar] [CrossRef]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant. biomaterials: A comprehensive review. World J. Clin. Cases WJCC 2015, 3, 52. [Google Scholar] [CrossRef]
- Salahinejad, E.; Hadianfard, M.J.; Macdonald, D.D.; Sharifi-Asl, S.; Mozafari, M.; Walker, K.J.; Rad, A.T.; Madihally, S.V.; Tayebi, L. In vitro electrochemical corrosion and cell viability studies on nickel-free stainless steel orthopedic implants. PLoS ONE 2013, 8, e61633. [Google Scholar] [CrossRef]
- Sivakumar, M.; Kumar Dhanadurai, K.S.; Rajeswari, S.; Thulasiraman, V. Failures in stainless steel orthopaedic implant devices: A survey. J. Mater. Sci. Lett. 1995, 14, 351–354. [Google Scholar] [CrossRef]
- Beddoes, J.; Bucci, K. The influence of surface condition on the localized corrosion of 316L stainless steel orthopaedic implants. J. Mater. Sci. Mater. Med. 1999, 10, 389–394. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [Green Version]
- Finšgar, M.; Uzunalić, A.P.; Stergar, J.; Gradišnik, L.; Maver, U. Novel chitosan/diclofenac coatings on medical grade stainless steel for hip replacement applications. Sci. Rep. 2016, 6, 26653. [Google Scholar] [CrossRef]
- Bayón, R.; Igartua, A.; González, J.J.; De Gopegui, U.R. Influence of the carbon content on the corrosion and tribocorrosion performance of Ti-DLC coatings for biomedical alloys. Tribol. Int. 2015, 88, 115–125. [Google Scholar] [CrossRef]
- Fojt, J.; Joska, L.; Málek, J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros. Sci. 2013, 71, 78–83. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Beline, T.; Ribeiro, A.L.R.; Rangel, E.C.; da Cruz, N.C.; Landers, R.; Faverani, L.P.; Vaz, L.G.; Fais, L.M.; Vicente, F.B.; et al. Development of binary and ternary titanium alloys for dental implants. Dent. Mater. 2017, 33, 1244–1257. [Google Scholar] [CrossRef] [Green Version]
- Mjöberg, B.; Hellquist, E.; Mallmin, H.; Lindh, U. Aluminum, Alzheimer’s disease and bone fragility. Acta Orthop. Scand. 1997, 68, 511–514. [Google Scholar] [CrossRef]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol. 2017, 40, 30–36. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Singh, R.; Dahotre, N.B. Corrosion degradation and prevention by surface modification of biometallic materials. J. Mater. Sci. Mater. Med. 2007, 18, 725–751. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloy. Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Y.; Xiong, J.; Hodgson, P.D.; Wen, C.E. Porous TiNbZr alloy scaffolds for biomedical applications. Acta Biomater. 2009, 5, 3616–3624. [Google Scholar] [CrossRef]
- Ramírez, G.; Rodil, S.E.; Arzate, H.; Muhl, S.; Olaya, J.J. Niobium based coatings for dental implants. Appl. Surf. Sci. 2011, 257, 2555–2559. [Google Scholar] [CrossRef]
- Reyes, K.M.; Kuromoto, N.K.; Claro, A.A.; Marino, C.E.B. Electrochemical stability of binary TiNb for biomedical applications. Mater. Res. Express 2017, 4, 075402. [Google Scholar] [CrossRef]
- Kapnisis, K.; Constantinou, M.; Kyrkou, M.; Nikolaou, P.; Anayiotos, A.; Constantinides, G. Nanotribological response of aC: H coated metallic biomaterials: The cases of stainless steel, titanium, and niobium. J. Appl. Biomater. Funct. Mater. 2018, 16, 230–240. [Google Scholar]
- Ou, K.L.; Weng, C.C.; Lin, Y.H.; Huang, M.S. A promising of alloying modified beta-type Titanium-Niobium implant for biomedical applications: Microstructural characteristics, in vitro biocompatibility and antibacterial performance. J. Alloy. Compd. 2017, 697, 231–238. [Google Scholar] [CrossRef]
- Tavares, A.M.G.; Fernandes, B.S.; Souza, S.A.; Batista, W.W.; Cunha, F.G.C.; Landers, R.; Macedo, M.C.S.S. The addition of Si to the Ti–35Nb alloy and its effect on the corrosion resistance, when applied to biomedical materials. J. Alloy. Compd. 2014, 591, 91–99. [Google Scholar] [CrossRef]
- Lee, C.; Ju, C.-P.; Chern Lin, J. Structure–property relationship of cast Ti–Nb alloys. J. Oral Rehab. 2002, 29, 314–322. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Panaino, J.V.P.; Santos, I.D.; Araujo, L.S.; Mei, P.R.; De Almeida, L.H.; Nunes, C.A. Characterization of a new beta titanium alloy, Ti–12Mo–3Nb, for biomedical applications. J. Alloy. Compd. 2012, 536, S208–S210. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.M.A.; Altaf, K.; Hussain, P.; Prakash, C.; Hastuty, S.; Rao, T.V.V.L.N.; Subramaniam, K. Investigation of alloy composition and sintering parameters on the corrosion resistance and microhardness of 316L Stainless Steel alloy. In Advances in Manufacturing II; Gapiński, B., Szostak, M., Ivanov, V., Eds.; Springer: Berlin, Germany, 2019; pp. 532–541. [Google Scholar]
- Bagliuk, G. Properties and structure of sintered boron containing carbon steels. In Sintering-Methods and Products; Shatokha, V., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Bayraktaroglu, E.; Gulsoy, H.O.; Gulsoy, N.; Er, O.; Kilic, H. Effect of boron addition on injection molded 316L stainless steel: Mechanical, corrosion properties and in vitro bioactivity. Bio Med. Mater. Eng. 2012, 22, 333–349. [Google Scholar]
- Kurgan, N. Effects of sintering atmosphere on microstructure and mechanical property of sintered powder metallurgy 316L stainless steel. Mater. Des. (1980–2015) 2013, 52, 995–998. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.; Majdi, A.; Mufti, R.A.; Hastuty, S.; Hussain, M.; Shehzad, N.; Baig, Z.; Aliyu, A.; Azeez, A. An Efficient Approach for Nitrogen Diffusion and Surface Nitriding of Boron-Titanium Modified Stainless Steel Alloy for Biomedical Applications. Metals 2019, 9, 755. [Google Scholar] [CrossRef]
- Chao, Z.; Yaomu, X.; Chufeng, L.; Conghua, L. The effect of mucin, fibrinogen and IgG on the corrosion behaviour of Ni–Ti alloy and stainless steel. Biometals 2017, 30, 367–377. [Google Scholar] [CrossRef]
- Hussein, M.A.; Yilbas, B.; Kumar, A.M.; Drew, R.; Al-Aqeeli, N. Influence of Laser Nitriding on the Surface and Corrosion Properties of Ti-20Nb-13Zr Alloy in Artificial Saliva for Dental Applications. J. Mater. Eng. Perform. 2018, 27, 4655–4664. [Google Scholar] [CrossRef]
- Nassar, A.E.; Nassar, E.E. Properties of aluminum matrix Nano composites prepared by powder metallurgy processing. J. King Saud Univ. Eng. Sci. 2017, 29, 295–299. [Google Scholar] [CrossRef] [Green Version]
- Čapek, J.; Stehlíková, K.; Michalcová, A.; Msallamová, Š.; Vojtěch, D. Microstructure, mechanical and corrosion properties of biodegradable powder metallurgical Fe-2 wt % X (X= Pd, Ag and C) alloys. Mater. Chem. Phys. 2016, 181, 501–511. [Google Scholar] [CrossRef]
- Singh, A.V.; Jahnke, T.; Xiao, Y.; Wang, S.; Yu, Y.; David, H.; Richter, G.; Laux, P.; Luch, A.; Srivastava, A.; et al. Peptide-Induced Biomineralization of Tin Oxide (SnO2) Nanoparticles for Antibacterial Applications. J. Nanosci. Nanotech. 2019, 19, 5674–5686. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; Von Der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]







| Alloy | Green Density | Standard Deviation | Sintered Density | Standard Deviation | Relative Density |
|---|---|---|---|---|---|
| S1 | 6.500 g/cm3 | 0.112 | 7.575 g/cm3 | 0.063 | 95.88% |
| S2 | 6.370 g/cm3 | 0.0432 | 7.411 g/cm3 | 0.023 | 93.81% |
| S3 | 6.240 g/cm3 | 0.057 | 7.367 g/cm3 | 0.051 | 93.25% |
| S4 | 6.160 g/cm3 | 0.035 | 7.285 g/cm3 | 0.092 | 92.21% |
| S5 | 6.080 g/cm3 | 0.029 | 7.190 g/cm3 | 0.028 | 91.01% |
| Sample | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|
| Porosity (%) | 4.10 | 5.68 | 6.20 | 7.36 | 8.60 |
| S.No | Sample | Weight (g) before Immersion | Weight (g) after Immersion | Δ m (g) |
|---|---|---|---|---|
| 1 | S1 | 17.310 | 17.306 | 0.004 |
| 2 | S2 | 18.130 | 18.127 | 0.003 |
| 3 | S3 | 18.190 | 18.188 | 0.002 |
| 4 | S4 | 17.260 | 18.258 | 0.002 |
| 5 | S5 | 18.250 | 18.248 | 0.002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Abdul Rani, A.M.; Mufti, R.A.; Azam, F.I.; Hastuty, S.; Baig, Z.; Hussain, M.; Shehzad, N. The Influence of Nitrogen Absorption on Microstructure, Properties and Cytotoxicity Assessment of 316L Stainless Steel Alloy Reinforced with Boron and Niobium. Processes 2019, 7, 506. https://doi.org/10.3390/pr7080506
Ali S, Abdul Rani AM, Mufti RA, Azam FI, Hastuty S, Baig Z, Hussain M, Shehzad N. The Influence of Nitrogen Absorption on Microstructure, Properties and Cytotoxicity Assessment of 316L Stainless Steel Alloy Reinforced with Boron and Niobium. Processes. 2019; 7(8):506. https://doi.org/10.3390/pr7080506
Chicago/Turabian StyleAli, Sadaqat, Ahmad Majdi Abdul Rani, Riaz Ahmad Mufti, Farooq I. Azam, Sri Hastuty, Zeeshan Baig, Murid Hussain, and Nasir Shehzad. 2019. "The Influence of Nitrogen Absorption on Microstructure, Properties and Cytotoxicity Assessment of 316L Stainless Steel Alloy Reinforced with Boron and Niobium" Processes 7, no. 8: 506. https://doi.org/10.3390/pr7080506
APA StyleAli, S., Abdul Rani, A. M., Mufti, R. A., Azam, F. I., Hastuty, S., Baig, Z., Hussain, M., & Shehzad, N. (2019). The Influence of Nitrogen Absorption on Microstructure, Properties and Cytotoxicity Assessment of 316L Stainless Steel Alloy Reinforced with Boron and Niobium. Processes, 7(8), 506. https://doi.org/10.3390/pr7080506