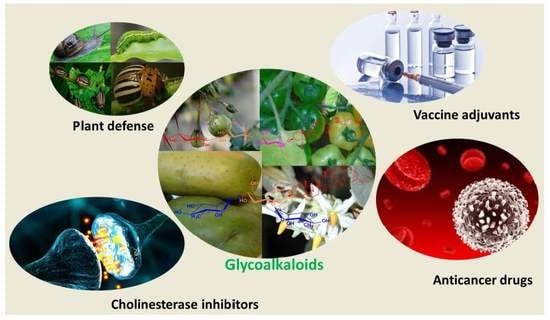
Glycoalkaloids: Structure, Properties, and Interactions with Model Membrane Systems
Abstract
:1. Introduction
2. Origin, Structure, and General Properties of Glycoalkaloids
2.1. Origin of Glycoalkaloids
2.2. Structure of Glycoalkaloids
2.3. General Properties of Glycoalkaloids
2.4. Anti-Cancer Properties of Glycoalkaloids
2.5. Use as a Vaccine Adjuvant
3. Interaction of Glycoalkaloids with Liposomes
4. Interaction of Glycoalkaloids with Monolayers at the Air–Water Interface
5. Summary of Key Results
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mecke, A.; Uppuluri, S.; Sassanella, T.M.; Leeb, D.-K.; Ramamoorthy, A.; Baker, J.A., Jr.; Orra, B.G.; Banaszak Holl, M.M. Direct observation of lipid bilayer disruption by poly(amidoamine) dendrimers. Chem. Phys. Lipids 2004, 132, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Xing, Y.; Liu, Y.; Zhang, Y.; Shen, X.; Li, X.; Miao, X.; Feng, Z.; Peng, X.; Qin, S. Fungicidal effect of chitosan via inducing membrane disturbance against Ceratocystis fimbriata. Carb. Polym. 2018, 192, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Shai, Y. Exploring peptide membrane interaction using surface plasmon resonance: Differentiation between pore formation versus membrane disruption by lytic peptides. Biochemistry 2003, 42, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Roddick, J.G. Steroidal glycoalkaloids: Nature and consequences of bioactivity. In Saponins Used in Traditional and Modern Medicine. Advances in Experimental Medicine and Biology; Waller, G.R., Yamasaki, K., Eds.; Springer: Boston, MA, USA, 1996; Volume 404, pp. 277–295. [Google Scholar]
- Friedman, M. Tomato glycoalkaloids: role in the plant and in the diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef]
- Ohvo-Rekilä, H.; Ramstedt, B.; Leppimäki, P.; Slotte, J.P. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002, 41, 66–97. [Google Scholar] [CrossRef]
- Lu, M.-K.; Shih, Y.-W.; Chien, T.-T.C.; Fang, L.-H.; Huang, H.-C.; Chen, P.-S. α-Solanine inhibits human melanoma cell migration and invasion by reducing matrix metalloproteinase-2/9 activities. Biol. Pharm. Bull. 2010, 33, 1685–1691. [Google Scholar] [CrossRef]
- Shih, Y.-W.; Chen, P.-S.; Wu, C.-H.; Jeng, Y.-F.; Wang, C.-J. α-Chaconine-reduced metastasis involves a PI3K/Akt signaling pathway with downregulation of NF-κB in human lung adenocarcinoma A549 cells. J. Agric. Food Chem. 2007, 55, 11035–11043. [Google Scholar] [CrossRef]
- Roddick, J.G. The acetylcholinesterase-inhibitory activity of steroidal glycoalkaloids and their aglycones. Phytochemistry 1989, 28, 2631–2634. [Google Scholar] [CrossRef]
- Benilova, I.V.; Arkhypova, V.N.; Dzyadevych, S.V.; Jaffrezic-Renault, N.; Martelet, C.; Soldatkin, A.P. Kinetics of human and horse sera cholinesterases inhibition with solanaceous glycoalkaloids: Study by potentiometric biosensor. Pesticide Biochem. Physiol. 2006, 86, 203–210. [Google Scholar] [CrossRef]
- Sucha, L.; Tomsik, P. The steroidal glycoalkaloids from Solanaceae: Toxic effect, antitumour activity and mechanism of action. Planta Med. 2016, 82, 379–387. [Google Scholar] [CrossRef]
- Jiang, Q.-W.; Chen, M.-W.; Cheng, K.-J.; Yu, P.-Z.; Wei, X.; Shi, Z. Therapeutic potential of steroidal alkaloids in cancer and other diseases. Med. Res. Rev. 2016, 36, 119–143. [Google Scholar] [CrossRef]
- Lee, S.T.; Wong, P.-F.; Hooper, J.D.; Mustafa, M.R. Alpha-tomatine synergises with paclitaxel to enhance apoptosis of androgen-independent human prostate cancer PC-3 cells in vitro and in vivo. Phytomedicine 2013, 20, 1297–1305. [Google Scholar] [CrossRef]
- Liang, C.-H.; Shiu, L.-Y.; Chang, L.-C.; Sheu, H.-M.; Tsai, E.-M.; Kuo, K.-W. Solamargine enhances HER2 expression and increases the susceptibility of human lung cancer H661 and H69 cells to Trastuzumab and Epirubicin. Chem. Res. Toxicol. 2008, 21, 393–399. [Google Scholar] [CrossRef]
- Sarmoko, A.; Putri, D.D.P.; Hermawan, A.; Meiyanto, E. Combination of Solanum nigrum L. herb ethanolic extract and Doxorubicin performs synergism on T47D breast cancer cells. Indones. J. Cancer Chemoprevent. 2010, 1, 78–84. [Google Scholar]
- Shiu, L.Y.; Chang, L.C.; Liang, C.H.; Huang, Y.S.; Sheu, H.M.; Kuo, K.W. Solamargine induces apoptosis and sensitizes breast cancer cells to cisplatin. Food Chem. Toxicol. 2007, 45, 2155–2164. [Google Scholar] [CrossRef]
- Morrow, W.J.W.; Yang, Y.-W.; Sheikh, N.A. Immunobiology of the tomatine adjuvant. Vaccine 2004, 22, 2380–2384. [Google Scholar] [CrossRef]
- Heal, K.G.; Sheikh, N.A.; Hollingdale, M.R.; Morrow, W.J.W.; Taylor-Robinson, A.W. Potentiation by a novel alkaloid glycoside adjuvant of a protective cytotoxic T cell immune response specific for a preerythrocytic malaria vaccine candidate antigen. Vaccine 2001, 19, 4153–4161. [Google Scholar] [CrossRef]
- Heal, K.G.; Taylor-Robinson, A.W. Tomatine Adjuvantation of protective immunity to a major pre-erythrocytic vaccine candidate of malaria is mediated via CD8+ T cell release of IFN-γ. J. Biomed. Biotech. 2010, 834326. [Google Scholar]
- Milner, S.E.; Brunton, N.P.; Jones, P.W.; O’ Brien, N.M.; Collins, S.G.; Maguire, A.R. Bioactivities of glycoalkaloids and their aglycones from Solanum species. J. Agric. Food Chem. 2011, 59, 3454–3484. [Google Scholar] [CrossRef]
- Percival, G.C.; Dixon, G.R. Glycoalkaloids. In Handbook of Plant and Fungal Toxins; Felix D’Mello, J.P., Ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 19–35. [Google Scholar]
- Friedman, M.; Dao, L. Distribution of glycoalkaloids in potato plants and commercial potato products. J. Agric. Food Chem. 1992, 40, 419–442. [Google Scholar] [CrossRef]
- Bajaj, K.L.; Kaur, G.; Chadha, M.L. Glycoalkaloid content and other chemical constituents of the fruits of some eggplant (Solanum melongena. L.) varieties. J. Plant Foods 1979, 3, 163–168. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Jarych-Szyszka, M.; Lisińska, G. Changes in glycoalkaloids content of potatoes destined for consumption. Food Chem. 2008, 106, 706–711. [Google Scholar] [CrossRef]
- Fitzpatrick, T.J.; McDermott, J.A.; Osman, S.F. Evaluation of injured commercial potato samples for total glycoalkaloid content. J. Food Sci. 1978, 43, 1417–1418. [Google Scholar] [CrossRef]
- Omayio, D.G.; Abong, G.O.; Okoth, M.W. A review of occurrence of glycoalkaloids in potato and potato products. Curr. Res. Nutr. Food Sci. 2016, 4, 195–202. [Google Scholar] [CrossRef]
- Kalinowska, M.; Zimowski, J.; Pączkowski, C.; Wojciechowski, Z.A. The formation of sugar chains in triterpenoid saponins and glycoalkaloids. Phytochem. Rev. 2005, 4, 237–257. [Google Scholar] [CrossRef]
- Roddick, J.G. Effect of α-tomatine on the integrity and biochemical activities of isolated plant cell organelles. J. Exp. Bot. 1978, 29, 1371–1381. [Google Scholar] [CrossRef]
- Friedman, M. Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef]
- Tingey, W.M. Glycoalkaloids as pest resistance factors. Am. Potato J. 1984, 61, 157–167. [Google Scholar] [CrossRef]
- Balandrin, M.F. Commercial Utilization of Plant-Derived Saponins: An Overview of Medicinal, Pharmaceutical, and Industrial Applications. In Saponins Used in Traditional and Modern Medicine. Advances in Experimental Medicine and Biology; Waller, G.R., Yamasaki, K., Eds.; Springer: Boston, MA, USA, 1996; Volume 404, pp. 1–14. [Google Scholar]
- Ito, S.; Ihara, T.; Tamura, H.; Tanaka, S.; Ikeda, T.; Kajihara, H.; Dissanayake, C.; Abdel-Motaald, F.F.; El-Sayed, M.A. α-Tomatine, the major saponin in tomato, induces programmed cell death mediated by reactive oxygen species in the fungal pathogen Fusarium oxysporum. FEBS Lett. 2007, 581, 3217–3222. [Google Scholar] [CrossRef]
- Sandrock, R.W.; Van Ette, H.D. Fungal sensitivity to and enzymatic degradation of the phytoanticipin α-tomatine. FEBS Lett. 2007, 581, 3217–3222. [Google Scholar] [CrossRef]
- Rubio, M.R.; Espinosa, A.P.; Lairini, K.; Arjona, T.R.; Dipietro, A.; Anaya, N. Metabolism of the tomato saponin α-tomatine by phytopathogenic fungi. Stud. Nat. Prod. Chem. 2001, 25, 293–326. [Google Scholar]
- Smith, D.B.; Roddick, J.G.; Jones, J.L. Synergism between the potato glycoalkaloids α-chaconine and α-solanine in inhibition of snail feeding. Phytochemistry 2001, 57, 229–234. [Google Scholar] [CrossRef]
- Zhang, T.M.; Mitchell, B.K. Components of the tomato leaf homogenate suppress responses from Galeal chemoreceptors of the adult Colorado beetle. Physiol. Entomol. 1997, 22, 291–296. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Harden, L.A. Preparation and characterization of acid hydrolysis products of the tomato glycoalkaloid α-tomatine. J. Agric. Food Chem. 1998, 46, 2096–2210. [Google Scholar] [CrossRef]
- Mensinga, T.T.; Sips, A.J.A.M.; Rompelberg, C.J.M.; van Twillert, K.; Meulenbelt, J.; van den Top, H.; van Egmond, H.P. Potato glycoalkaloids and adverse effects in humans: An ascending dose study. Regul. Toxicol. Pharmacol. 2005, 41, 66–72. [Google Scholar] [CrossRef]
- Morris, S.C.; Lee, T.H. The toxicity and teratogenicity of Solanaceae glycoalkaloids, particularly those of the potato (Solanum tuberosum): A review. Food Technol. Aust. 1984, 36, 118–124. [Google Scholar]
- Roddick, J.G.; Rijnenberg, A.L.; Osman, S.F. Synergistic interaction between potato glycoalkaloids α-solanine and α-chaconine in relation to destabilization of cell membranes: Ecological implications. J. Chem. Ecol. 1988, 14, 889–902. [Google Scholar] [CrossRef]
- Yamashoji, S.; Matsuda, T. Synergistic cytotoxicity induced by α-solanine and α-chaconine. Food Chem. 2013, 141, 669–674. [Google Scholar] [CrossRef]
- Roddick, J.G.; Rijnenberg, A.L.; Weissenberg, M. Membrane-disrupting properties of the steroidal glycoalkaloids solasonine and solamargine. Phytochemistry 1990, 29, 1513–1518. [Google Scholar] [CrossRef]
- Roddick, J.G. Complex formation between solanaceous steroidal glycoalkaloids and free sterols in vitro. Phytochemistry 1979, 18, 1467–1470. [Google Scholar] [CrossRef]
- Elias, P.M.; Friend, D.S.; Goerke, J. Membrane sterol heterogeneity: Freeze fracture detection with saponins and filipin. J. Histochem. Cytochem. 1979, 27, 1247–1260. [Google Scholar] [CrossRef]
- Koehler, J.K.; Clark, J.M.; Smith, D. Freeze-fracture observations on mammalian oocytes. Dev. Dynam. 1985, 174, 317–329. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Beknke, O.; Tranum-Jensen, J.; van Deurs, B. Filipin as a cholesterol probe. I. Morphology of filipin-cholesterol interaction in lipid model systems. Eur. J. Cell Biol. 1984, 35, 189–199. [Google Scholar]
- De Groot, C.; Müller-Goymann, C.C. Saponin interactions with model membrane systems – Langmuir monolayer studies, hemolysis and formation of ISCOMs. Planta Med. 2016, 82, 1496–1512. [Google Scholar] [CrossRef]
- Yang, S.-A.; Paek, S.-H.; Kozukue, N.; Lee, K.R.; Kim, J.-A. α-Chaconine, a potato glycoalkaloid, induces apoptosis of HT-29 human colon cancer cells through caspase-3 activation and inhibition of ERK1/2 phosphorylation. Food Chem. Toxicol. 2006, 44, 839–846. [Google Scholar] [CrossRef]
- Kúdelová, J.; Seifrtová, M.; Suchá, L.; Tomšík, P.; Havelek, R.; Řezáčová, M. Alpha-tomatine activates cell cycle checkpoints in the absence of DNA damage in human leukemic MOLT-4 cells. J. Appl. Biomed. 2013, 11, 93–103. [Google Scholar] [CrossRef]
- Shieh, J.-M.; Cheng, T.-W.; Shi, M.-D.; Wu, P.-F.; Chen, Y.; Ko, S.-C.; Shih, Y.-W. α-Tomatine suppresses invasion and migration of human non-small cell lung cancer NCI-H460 cells through inactivating FAK/PI3K/Akt signaling pathway and reducing binding activity of NF-κB. Cell Biochem. Biophys. 2011, 60, 297–310. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Lee, S.-U.; Kim, H.-J.; Lee, I.-S.; Byun, J.-O.; Kozukue, N. Tomatine-containing green tomato extracts inhibit growth of human breast, colon, liver, and stomach cancer cells. J. Agric. Food Chem. 2009, 57, 5727–5733. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Soffers, A.E.M.F.; Dusemund, B.; Rietjens, I.M.C.M. Alkaloids in the human food chain – Natural occurrence and possible adverse effects. Mol. Nutr. Food Res. 2012, 56, 30–52. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, J.-B.; Kozukue, N.; Kim, H.-J.; Nishitani, Y.; Zhang, L.; Mizuno, M.; Levin, C.E.; Friedman, M. Structure—activity relationships of α-, β1-, γ-, and δ-tomatine and tomatidine against human breast (MDA-MB-231), gastric (KATO-III), and prostate (PC3) cancer cells. J. Agric. Food Chem. 2012, 60, 3891–3899. [Google Scholar] [CrossRef]
- Friedman, M.; Lee, K.-R.; Kim, H.-J.; Lee, I.-S.; Kozukue, N. Anticarcinogenic effects of glycoalkaloids from potatoes against human cervical, liver, lymphoma, and stomach cancer cells. J. Agric. Food Chem. 2005, 53, 6162–6169. [Google Scholar] [CrossRef]
- Nakamura, T.; Komori, C.; Lee, Y.-Y.; Hashimoto, F.; Yahara, S.; Nohara, T.; Ejima, A. Cytotoxic activities of Solanum steroidal glycosides. Biol. Pharm. Bull. 1996, 19, 564–566. [Google Scholar] [CrossRef]
- Sucha, L.; Hroch, M.; Rezacova, R.; Rudolf, E.; Havelek, R.; Sispera, L.; Cmielova, J.; Kohlerova, R.; Bezrouk, A.; Tomsik, P. The cytotoxic effect of α-tomatine in MCF-7 human adenocarcinoma breast cancer cells depends on its interaction with cholesterol in incubation media and does not involve apoptosis induction. Oncol. Rep. 2013, 30, 2593–2602. [Google Scholar] [CrossRef]
- Lee, K.-R.; Kozukue, N.; Han, J.-S.; Park, J.-H.; Chang, E.-Y.; Baek, E.-J.; Chang, J.-S.; Friedman, M. Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. J. Agric. Food Chem. 2004, 52, 2832–2839. [Google Scholar] [CrossRef]
- Wei, G.; Wang, J.; Du, Y. Total synthesis of solamargine. Bioorg. Med. Chem. Lett. 2011, 21, 2930–2933. [Google Scholar] [CrossRef]
- Li, N.; Cao, L.; Wang, Y.-R.; Tao, X.-Q.; Ding, G.; Wang, Z.-R.; Xiao, W. Induction of Solasonine on Apoptosis of Human Breast Cancer Bcap-37 Cells through Mitochondria-Mediated Pathway. Chin. Herb. Med. 2016, 8, 164–172. [Google Scholar] [CrossRef]
- Lee, S.-T.; Wong, P.-F.; Cheah, S.-C.; Mustafa, M.R. Alpha-tomatine induces apoptosis and inhibits nuclear factor-kappa B activation on human prostatic adenocarcinoma PC-3 cells. PLoS ONE 2011, 6, e18915. [Google Scholar] [CrossRef]
- Zuber, T.; Holm, D.; Byrne, P.; Ducreux, L.; Taylor, M.; Kaiseraand, M.; Stushnoff, C. Optimization of in vitro inhibition of HT-29 colon cancer cell cultures by Solanum tuberosum L. extracts. Food Funct. 2015, 6, 72–83. [Google Scholar] [CrossRef]
- Munari, C.C.; de Oliveira, P.F.; Leandro, L.F.; Pimenta, L.M.; Ferreira, N.H.; Carvalho da Costa, J.; Bastos, J.K.; Tavares, D.C. In Vivo Assessment of Genotoxic, Antigenotoxic and Anticarcinogenic Activities of Solanum lycocarpum Fruits Glycoalkaloidic Extract. PLoS ONE 2014, 9, e111999. [Google Scholar] [CrossRef]
- Wu, C.-H.; Liang, C.-H.; Shiu, L.-Y.; Chang, L.-C.; Lin, T.-S.; Lan, C.-C.E.; Tsai, J.-C.; Wong, T.-W.; Wei, K.-J.; Lin, Y.T.-K.; et al. Solanum incanum extract (SR-T100) induces human cutaneous squamous cell carcinoma apoptosis through modulating tumor necrosis factor receptor signaling pathway. J. Dermatol. Sci. 2011, 63, 83–92. [Google Scholar] [CrossRef]
- Kim, S.P.; Nam, S.H.; Friedman, M. The Tomato Glycoalkaloid α-Tomatine Induces Caspase-Independent Cell Death in Mouse Colon Cancer CT-26 Cells and Transplanted Tumors in Mice. J. Agric. Food Chem. 2015, 63, 1142–1150. [Google Scholar] [CrossRef]
- Rajananthanan, P.; Attard, G.S.; Sheikh, N.A.; Morrow, W.J.W. Novel aggregate structure adjuvants modulate lymphocyte proliferation and Th1 and Th2 cytokine profiles in ovalbumin immunized mice. Vaccine 1999, 18, 140–152. [Google Scholar] [CrossRef]
- Sheikh, N.A.; Rajananthanan, P.; Attard, G.S.; Morrow, W.J.W. Generation of antigen specific CD8+ cytotoxic T cells following immunization with soluble protein formulated with novel glycoside adjuvants. Vaccine 1999, 17, 2974–2982. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Sun, F.; Han, H.; Zhang, X.; Fan, Y.; Tai, G.; Zhou, Y. In-vivo antimalarial activities of glycoalkaloids isolated from Solanaceae plants. Pharm. Biol. 2010, 48, 1018–1024. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Sheikh, N.A.; Morrow, W.J.W. The ultrastructure of tomatine adjuvant. Biomaterials 2002, 23, 4677–4686. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Wua, C.-A.; Morrow, W.J.W. The apoptotic and necrotic effects of tomatine adjuvant. Vaccine 2004, 22, 2316–2327. [Google Scholar] [CrossRef]
- Roddick, J.G.; Drysdale, R.B. Destabilization of liposome membranes by the steroidal glycoalkaloid α-tomatine. Phytochemistry 1984, 23, 543–547. [Google Scholar] [CrossRef]
- Roddick, J.G.; Rijnenberg, A.L.; Weissenberg, M. Alterations to the permeability of liposome membranes by the solasodine-based glycoalkaloids solasonine and solamargine. Phytochemistry 1992, 31, 1951–1954. [Google Scholar] [CrossRef]
- Roddick, J.G.; Rijnenberg, A.L. Synergistic interaction between the potato glycoalkaloids α-solanine and α-chaconine in relation to lysis of phospholipid/sterol liposomes. Phytochemistry 1987, 26, 1325–1328. [Google Scholar] [CrossRef]
- Roddick, J.G.; Weissenberg, M.; Leonard, A.L. Membrane disruption and enzyme inhibition by naturally-occurring and modified chacotriose-containing Solanum steroidal glycoalkaloids. Phytochemistry 2001, 56, 603–610. [Google Scholar] [CrossRef]
- Keukens, E.A.J.; de Vrije, T.; Fabrie, C.H.J.P.; Demel, R.A.; Jongen, W.M.F.; de Kruijff, B. Dual specificity of sterol-mediated glycoalkaloid induced membrane disruption. Biochim. Biophys. Acta 1992, 1110, 127–136. [Google Scholar] [CrossRef]
- Keukens, E.A.J.; de Vrije, T.; van den Boom, C.; de Waard, P.; Plasman, H.H.; Thiel, F.; Chupin, V.; Jongen, W.M.F.; de Kruijff, B. Molecular basis of glycoalkaloid induced membrane disruption. Biochim. Biophys. Acta 1995, 1240, 216–228. [Google Scholar] [CrossRef] [Green Version]
- Keukens, E.A.J.; de Vrije, T.; Jansen, A.M.; de Boer, H.; Janssen, M.; de Kroon, A.I.P.M.; Jongen, W.M.F.; de Kruijff, B. Glycoalkaloids selectively permeabilize cholesterol containing biomembranes. Biochim. Biophys. Acta 1996, 1279, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Giner-Casaresa, J.J.; Brezesinski, G.; Möhwald, H. Langmuir monolayers as unique physical models. Curr. Opin. Colloid Interface Sci. 2014, 19, 176–182. [Google Scholar] [CrossRef]
- Stine, K.J.; Hercules, R.K.; Duff, J.D.; Walker, B.W. Interaction of the glycoalkaloid tomatine with DMPC and sterol monolayers studied by surface pressure measurements and Brewster angle microscopy. J. Phys. Chem. B 2006, 110, 22220–22229. [Google Scholar] [CrossRef]
- Stine, K.J. Brewster Angle Microscopy. In Supramolecular Chemistry: From Molecules to Nanomaterials; Steed, J.W., Gale, P.A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2012; pp. 589–618. [Google Scholar]
- Nandi, N.; Vollhardt, D. Effect of Molecular Chirality on the Morphology of Biomimetic Langmuir Monolayers. Chem. Rev. 2003, 103, 4033–4076. [Google Scholar] [CrossRef]
- Walker, B.W.; Manhanke, N.; Stine, K.J. Comparison of the interaction of tomatine with mixed monolayers containing phospholipid, egg sphingomyelin, and sterols. Biochim. Biophys. Acta 2008, 1778, 2244–2257. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowski, K.; Orczyk, M.; Gutberlet, T.; Brezesinski, G.; Geue, T.; Fontaine, P. On the interaction between digitonin and cholesterol in Langmuir monolayers. Langmuir 2016, 32, 9064–9073. [Google Scholar] [CrossRef]
- Orczyk, M.; Wojciechowski, K.; Brezesinski, G. Disordering effects of digitonin on phospholipid monolayers. Langmuir 2017, 33, 3871–3881. [Google Scholar] [CrossRef]
- Orczyk, M.; Wojciechowski, K. Unusual penetration of phospholipid mono- and bilayers by Quillaja bark saponin biosurfactant. Biochim. Biophys. Acta 2014, 1838, 1931–1940. [Google Scholar] [Green Version]
- Wojciechowski, K.; Orczyk, M.; Gutberlet, T.; Geue, T. Complexation of phospholipids and cholesterol by triterpenic saponins in bulk and in monolayers. Biochim. Biophys. Acta 2016, 1858, 363–373. [Google Scholar] [CrossRef]
- Frenkel, N.; Makky, A.; Sudji, I.R.; Wink, M.; Tanaka, M. Mechanistic investigation of interactions between steroidal saponin digitonin and cell membrane models. J. Phys. Chem. B 2014, 118, 14632–14639. [Google Scholar] [CrossRef]









| Glycoalkaloid | Cell Type | Dosage (g mL−1) | Outcome | Ref |
|---|---|---|---|---|
| α-chaconine | HT-29 human colon carcinoma | 5.0 | Apoptosis, ERK inhibition, caspase-3 activation | [49] |
| α-chaconine | HepG2 liver cancer | 10 | 94.9% reduction in MTT activity after 48 h | [55] |
| α-chaconine | AGS gastric cancer | 10 | 89.7% reduction in MTT activity after 48 h | [55] |
| α-chaconine | PC-6 lung cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 1.83 μg mL−1 | [56] |
| α-chaconine | MCF-7 breast cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 1.54 μg mL−1 | [56] |
| α-chaconine | NUGC-3 stomach cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 1.43 μg mL−1 | [56] |
| α-chaconine | SW620 colon cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 1.46 μg mL−1 | [56] |
| α-chaconine + α-solanine | HepG2 liver cancer | Combinations that add to 10 (5 + 5, 3 + 7, 7 + 3, 1 + 9, 9 + 1) | 9 + 1 combination is synergistic (94.7% reduction in MTT activity), others are antagonistic | [57] |
| α-chaconine + α-solanine | AGS gastric cancer | Combinations that add to 10 (5 + 5, 3 + 7, 7 + 3, 1 + 9, 9 + 1) | All combinations are synergistic, 87.6–89.0% reduction in MTT activity | [58] |
| α-chaconine and hydrolysis products | HT29 colon cancer cells | 0.1, 0.5, 1, 5, 10, 100 | Growth inhibition by MTT assay = 77.3% for α-chaconine, 48.5% for β1-chaconine, 53.6% for β2-chaconine, 12.8% for γ-chaconine, and 32.5% for solanidine (aglycone). (4 h, 10 μg mL−1, data also reported for 24 h, 48 h and other concentrations) | [58] |
| α-chaconine and hydrolysis products | HepG2 liver cancer cells | 0.1, 0.5, 1, 5, 10, 100 | Growth inhibition by MTT assay = 83.3% for α-chaconine, 44.0% for β1-chaconine, 37.8% for β2-chaconine, 76.3% for γ-chaconine, and 71.5% for solanidine (aglycone). (4 h, 10 μg mL−1, data also reported for 24 h, 48 h and other concentrations) | [58] |
| α-solamargine | HT29 colon cancer cells | 0.1, 0.5, 1, 5, 10, 100 | Growth inhibition by MTT assay = 71.8% (4 h, 10 μg mL−1, data also reported for 24 h, 48 h and other concentrations) | [58] |
| α-solamargine | HepG2 liver cancer cells | 0.1, 0.5, 1, 5, 10, 100 | Growth inhibition by MTT assay = 81.4% (4 h, 10 μg mL−1, data also reported for 24 h, 48 h and other concentrations) | [55] |
| α-solamargine | PC-6 lung cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 2.66 μg mL−1 | [56] |
| α-solamargine | MCF-7 breast cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 2.16 μg mL−1 | [56] |
| α-solamargine | NUGC-3 stomach cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 1.95 μg mL−1 | [56] |
| α-solamargine | SW620 colon cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 1.62 μg mL−1 | [56] |
| α-solamargine | HeLa cervical cancer | Not stated | IC50 (MTT assay) = 6.0 μg mL−1 | [59] |
| α-solamargine | A549 lung cancer | Not stated | IC50 (MTT assay) = 8.0 μg mL−1 | [59] |
| α-solamargine | MCF-7 breast cancer | Not stated | IC50 (MTT assay) = 2.1 μg mL−1 | [59] |
| α-solamargine | K562 chronic myelogenous leukemia | Not stated | IC50 (MTT assay) = 5.2 μg mL−1 | [59] |
| α-solamargine | HCT116 colon cancer | Not stated | IC50 (MTT assay) = 3.8 μg mL−1 | [59] |
| α-solamargine | U87 glioblastoma | Not stated | IC50 (MTT assay) = 3.2 μg mL−1 | [59] |
| α-solamargine | HepG2 liver cancer | Not stated | IC50 (MTT assay) = 2.5 μg mL−1 | [59] |
| α-solamargine | H661 large cell lung cancer | 0–10.4 | ED50 ([3H]thymidine DNA incorporation assay at 18 h) = 3.11 μg mL−1, increase in HER2 expression, evidence for apoptosis including chromatin condensation, DNA fragmentation and cell morphology changes | [14] |
| α-solamargine | H69 small cell lung cancer | 0–10.4 | ED50 ([3H]thymidine DNA incorporation assay at 18 h) = 5.02 μg mL−1, increase in HER2 expression, evidence for apoptosis including chromatin condensation, DNA fragmentation and cell morphology changes | [14] |
| α-solamargine | HBL-100 breast cancer cells | 0–217 | IC50 (MTS cell viability assay at 16 h) = 1.80 μg mL−1, evidence for apoptosis, upregulated TNFR-I, Fas, and TRADD, upregulate Bax and downregulate Bcl-2 and Bcl-xL, chromatin condensation, blebbing, and shrinkage | [16] |
| α-solamargine | ZR-75-1 breast cancer cells | 0–217 | IC50 (MTS cell viability assay at 16 h) = 2.60 μg mL−1, same as for HBL-100 cells | [16] |
| α-solamargine | SK-BR-3 breast cancer cells | 0–217 | IC50 (MTS cell viability assay at 16 h) = 1.87 μg mL−1, same as for HBL-100 cells | [16] |
| α-solamargine | Bcap-37 human breast cancer | 0–8.84 | IC50 (MTT assay after 24 h) = 5.61 μg mL−1, evidence for apoptosis similar but stronger as for α-solasonine | [60] |
| α-solamargine | MCF-7 human breast cancer | 0–8.84 | IC50 (MTT assay after 24 h) = 1.14 μg mL−1 | [60] |
| α-solamargine | A-431 epidermoid carcinoma | 0–8.84 | IC50 (MTT assay after 24 h) = 8.27 μg mL−1 | [60] |
| α-solanine | HepG2 liver cancer | 10 | 86.6% reduction in MTT activity after 48 h | [55] |
| α-solanine | AGS gastric cancer | 10 | 79.0% reduction in MTT activity after 48 h | [55] |
| α-solanine | PC-6 lung cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 15.70 μg mL−1 | [56] |
| α-solanine and hydrolysis products | HT29 colon cancer cells | 0.1, 0.5, 1, 5, 10, 100 | Growth inhibition by MTT assay = 81.7% for α-solanine, 51.4% for β2-solanine, 55.0% for solasodine (aglycone). (4 h, 10 μg mL−1, data also reported for 24 h, 48 h and other concentrations) | [58] |
| α-solanine and hydrolysis products | HepG2 liver cancer cells | 0.1, 0.5, 1, 5, 10, 100 | Growth inhibition by MTT assay = 80.9% for α-solanine, 13.3% for β2-solanine, 78.3% for solasodine (aglycone). (4 h, 10 μg mL−1, data also reported for 24 h, 48 h and other concentrations) | [58] |
| α-solasonine | PC-6 lung cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 14.4 μg mL−1 | [56] |
| α-solasonine | MCF-7 breast cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 9.70 μg mL−1 | [56] |
| α-solasonine | NUGC-3 stomach cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 12.10 μg mL−1 | [56] |
| α-solasonine | SW620 colon cancer | Not stated | Growth inhibition of 50% at 72 h (GI50) = 6.72 μg mL−1 | [56] |
| α-solasonine | Bcap-37 human breast cancer | 0–17.36 | IC50 (MTT assay after 24 h) = 19.64 μg mL−1, evidence for apoptosis, mitochondrial dysfunction and cytochrome c release, caspase-3 activity, Annexin V positive staining, downregulated BCl-2 and Bcl-xL and upregulated Bax | [60] |
| α-solasonine | MCF-7 human breast cancer | 0–17.36 | IC50 (MTT assay after 24 h) = 11.70 μg mL−1 | [60] |
| α-solasonine | A-431 epidermoid carcinoma | 0–17.36 | IC50 (MTT assay after 24 h) = 15.67 μg mL−1 | [60] |
| α-solasonine and aglycone | HT29 colon cancer cells | 0.1, 0.5, 1, 5, 10, 100 | Growth inhibition by MTT assay = 68.4%, 55% for solasodine (aglycone). (4 h, 10 μg mL−1, data also reported for 24 h, 48 h and other concentrations) | [58] |
| α-solasonine and aglycone | HepG2 liver cancer cells | 0.1, 0.5, 1, 5, 10, 100 | Growth inhibition by MTT assay = 79.3%. 78.3% for solasodine (aglycone). (4 h, 10 μg mL−1, data also reported for 24 h, 48 h and other concentrations) | [58] |
| α-tomatine | MOLT-4 human T-lymphoblastic leukemia | 1.03–4.12 | Caspase independent cell death, increase p53, increase in PUMA protein, increase p21WAFI/CIPI, activation of Chk2, cell cycle arrest in G1 phase, no change in viability at 1.03 μg mL−1 but 12% cell viability after 24 h for 4.12 μg mL−1 | [50] |
| α-tomatine | NCI-H460 (human lung large cell carcinoma) | up to 4.12 | Cytotoxic above 1.55 μg mL−1, reduce mitochondrial membrane potential, reduce GSH, increase reactive oxygen species, change in cell morphology to elongated, spindle, or shrunken, inactivate PI3K/Akt signaling, enhancing IκBα protein expression, reduce NF-κB DNA-binding activity, downregulate MMP-7, interfere with the rearrangement of the actin cytoskeleton, decrease expression of-FAK, inhibit cell invasion and migration. | [51] |
| α-tomatine | AGS stomach cancer | 10.34–51.7 | IC50 = 0.03 μg mL−1 by MTT assay after 48 h | [52] |
| α-tomatine | HepG2 liver cancer | 10.34–51.7 | IC50 = 43 μg mL−1 by MTT assay after 48 h | [52] |
| α-tomatine | Ht-29 colon cancer | 10.34–51.7 | IC50 = 0.03 μg mL−1 by MTT assay after 48 h | [52] |
| α-tomatine | MCF-7 breast cancer | 10.34–51.7 | IC50 = 5.07 μg mL−1 by MTT assay after 48 h | [52] |
| α-tomatine | LNCaP prostate cancer cells | 0.52–4.12 | EC50 = 2.74 ± 0.01 μg mL−1 for 24 h treatment | [52] |
| α-tomatine | MCF-7 human breast cancer | 1.034–9.306 | EC50 = 7.41 (72 h), no DNA damage, loss of ATP, microscopy shows the collapse of some cells, TEM shows sign of rapid necrosis | [55] |
| α-tomatine | PC-3 prostate cancer | 0.17–5.17 | EC50 (MTT assay after 24 h) = 1.73 μg mL−1. Evidence for apoptosis, Annexin V staining is positive, decrease in mitochondrial membrane potential, caspase-3, -8, and -9 activity, nF-kB activation inhibited, nuclear condensation | [61] |
| α-tomatine and hydrolysis products | MDA-MB-231 breast cancer | 1, 10, 50, 100 | IC50 = 26.4 ± 3.6 for α-tomatine, 82.3 ± 11.0 for β1-tomatine, 137.8 ± 16.6 for γ-tomatine, 84.5 ± 6.6 for δ-tomatine, and 336.5 ± 7.9 for tomatidine (all in μg mL−1, all 48 h) | [54] |
| α-tomatine and hydrolysis products | KATO-III gastric cancer | 1, 10, 50, 100 | IC50 = 16.4 ± 10.0 for α-tomatine, 77.1 ± 13.3 for β1-tomatine, 156.0 ± 8.4 for γ-tomatine, 150.4 ± 11.3 for δ-tomatine, and 623.0 ± 7.9 for tomatidine (all in μg mL−1, all 48 h) | [54] |
| α-tomatine and hydrolysis products | HT29 colon cancer cells | 0.1, 0.5, 1, 5, 10, 100 | Growth inhibition by MTT assay = 71.6% for α-tomatine, 51.3% for β1-tomatine, 17.5% for γ-tomatine, 26.3% for δ-tomatine and 11.4% for tomatidine (aglycone). (4 h, 10 μg mL−1, data also reported for 24 h, 48 h and other concentrations) | [58] |
| α-tomatine and hydrolysis products | HepG2 liver cancer cells | 0.1, 0.5, 1, 5, 10, 100 | Growth inhibition by MTT assay = 85.5% for α-tomatine, 80.9% for β1-tomatine, 17.8% for γ-tomatine, 52.8% for δ-tomatine and 28.8% for tomatidine (aglycone). (4 h, 10 μg mL−1, data also reported for 24 h, 48 h and other concentrations) | [58] |
| α-tomatine and hydrolysis | PC3 prostate cancer | 1, 10, 50, 100 | IC50 = 3.0 ± 0.3 for α-tomatine, 82.5 ± 9.6 for β1-tomatine, 103.2 ± 16.6 for γ-tomatine, 100.5 ± 5.0 for δ-tomatine, and 248.9 ± 11.2 for tomatidine (all in μg mL−1, all 48 h) | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nepal, B.; J. Stine, K. Glycoalkaloids: Structure, Properties, and Interactions with Model Membrane Systems. Processes 2019, 7, 513. https://doi.org/10.3390/pr7080513
Nepal B, J. Stine K. Glycoalkaloids: Structure, Properties, and Interactions with Model Membrane Systems. Processes. 2019; 7(8):513. https://doi.org/10.3390/pr7080513
Chicago/Turabian StyleNepal, Bishal, and Keith J. Stine. 2019. "Glycoalkaloids: Structure, Properties, and Interactions with Model Membrane Systems" Processes 7, no. 8: 513. https://doi.org/10.3390/pr7080513
APA StyleNepal, B., & J. Stine, K. (2019). Glycoalkaloids: Structure, Properties, and Interactions with Model Membrane Systems. Processes, 7(8), 513. https://doi.org/10.3390/pr7080513