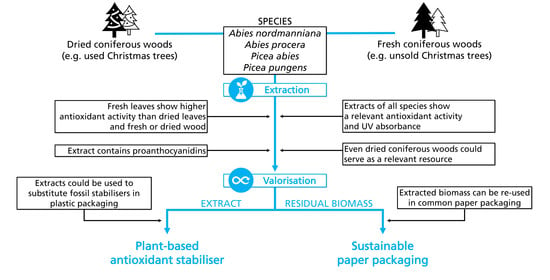
Characterisation of Bioactive Ingredients in Extracts of Fresh and Dried Coniferous Trees for the Development of Sustainable Packaging Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
2.2. Sample Preparation and Extraction
2.3. Determination of UV Absorbance
2.4. Determination of TAC via ABTS Radical Cation (ABTS•+) Scavenging Capacity Assay (ABTS Assay)
2.5. Determination of Total Phenolic Content (TPC) via Folin–Ciocalteu Assay
2.6. Acid–Butanol Assay (ABA)
2.7. Gas Chromatography Coupled with Mass Spectrometry (GC-MS)
2.8. Preparation and Analysis of the Paper Sheets
2.9. Statistical Interpretation
3. Results and Discussion
3.1. General Suitability
3.1.1. UV/Vis Absorbance
3.1.2. TAC and TPC
3.2. Analysis of Waste Valorisation: Utilisation of Christmas Trees after Private Usage
3.2.1. UV/Vis Absorbance
3.2.2. TAC and TPC
3.2.3. Extraction Optimisation
3.2.4. Production of Sustainable Paper Packaging Materials from Extraction Waste Products
3.3. Characterisation of the Extracts of Different Branch Fractions for Fresh and Dried Masses
3.3.1. UV/Vis Absorbance
3.3.2. TAC and TPC
3.3.3. Further Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wegmann, A.; Le Gal, A.; Müller, D. Antioxidantien. In Handbuch Kunststoff-Additive, 4., Vollständig Neu Bearbeitete Auflage; Maier, R.-D., Schiller, M., Eds.; Hanser: München, Germany, 2016; ISBN 978-3-446-22352-3. [Google Scholar]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar] [CrossRef] [PubMed]
- Kahl, R.; Kappus, H. Toxikologie der synthetischen Antioxidantien BHA und BHT im Vergleich mit dem natürlichen Antioxidans Vitamin E. Z. Lebensm. Unters. For. 1993, 196, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, M.A.; Wagner, J.; Jiménez, A. Migration study of carvacrol as natural antioxidant in High Density Polyethylene for active packaging. Food Addit. Contam. 2009, 26, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Detzel, A.; Kauertz, B.; Derreza-Greeven, C.; Reinhardt, J.; Kunze, S.; Krüger, M.; Fehrenbach, H.; Volz, S. Endbericht Untersuchung der Umweltwirkungen von Verpackungen aus Biologisch Abbaubaren Kunststoffen. 2012. Available online: http://www.uba.de/uba-info-medien/3986.html (accessed on 15 April 2020).
- Havelt, T.; Schmitz, M. Identifizierung und Charakterisierung bioaktiver Inhaltsstoffe in Thymian: 8. Tagung Arznei- und Gewürzpflanzenforschung, Bonn. Julius-Kühn-Archiv 2018, 112–114. [Google Scholar] [CrossRef]
- Havelt, T.; Brettschneider, S.; Do, X.T.; Korte, I.; Kreyenschmidt, J.; Schmitz, M. Sustainable Extraction and Characterisation of Bioactive Compounds from Horse Chestnut Seed Coats for the Development of Bio-Based Additives. Resources 2019, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Schutzgemeinschaft Deutscher Wald. Weihnachtsbaum in Zahlen und Fakten: Daten zum Weihnachtsbaum 2020. Available online: https://www.sdw.de/waldwissen/weihnachtsbaum/ (accessed on 22 January 2020).
- Geismann, U.; Tews, F.; Hauptverband der Deutschen Holzindustrie e.V. Deutsche kaufen in diesem Jahr 29,8 Millionen Weihnachtsbäume. Weihnachtsbaum 2019: Stabile Stückzahlen, Stabile Preise. 2019. Available online: https://www.holzindustrie.de/pressemitteilungen/2926/deutsche-kaufen-in-diesem-jahr-29-8-millionen-weihnachtsbaeume.html (accessed on 21 January 2020).
- Knebel, A.; Agentur für Erneuerbare Energien. Weihnachtsbäume Liefern Grüne Energie. 2015. Available online: https://www.unendlich-viel-energie.de/presse/pressemitteilungen/weihnachtsbaeume-liefern-gruene-energie (accessed on 20 January 2020).
- Bağcı, E.; Dığrak, M. Antimicrobial Activity of Essential Oils of some Abies (Fir) Species from Turkey. Flavour Frag. J. 1996, 11, 251–256. [Google Scholar] [CrossRef]
- Öncel, I.; Yurdakulol, E.; Keleş, Y.; Kurt, L.; Yıldız, A. Role of antioxidant defense system and biochemical adaptation on stress tolerance of high mountain and steppe plants. Acta Oecol. 2004, 26, 211–218. [Google Scholar] [CrossRef]
- Weber, P.; Bendich, A.; Schalch, W. Vitamin C and human health--a review of recent data relevant to human requirements. Int. J. Vitam. Nutr. Res. 1996, 66, 19–30. [Google Scholar]
- Charles, D.J. Natural Antioxidants. In Antioxidant Properties of Spices, Herbs and Other Sources; Charles, D.J., Ed.; Springer: New York, NY, USA, 2013; pp. 39–64. ISBN 978-1-4614-4309-4. [Google Scholar]
- Elmezughi, J.; Shittu, H.; Clements, C.; Edrada-Ebel, R.A.; Seidel, V.; Gray, A. Bioactive natural compounds from Prosopis africana and Abies nobili. J. App. Pharm. Sci. 2013. [Google Scholar] [CrossRef] [Green Version]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Kartnig, T.; Still, F.; Reinthaler, F. Antimicrobial activity of the essential oil of young pine shoots (Picea abies L.). J. Ethnopharmacol. 1991, 35, 155–157. [Google Scholar] [CrossRef]
- Hafizoglu, H.; Holmbom, B. Chemical composition of extractives from Abies nordmanniana. Holz Roh. Werkst. 1995, 53, 273–275. [Google Scholar] [CrossRef]
- Polle, A.; Chakrabarti, K.; Schürmann, W.; Rennenberg, H. Composition and Properties of Hydrogen Peroxide Decomposing Systems in Extracellular and Total Extracts from Needles of Norway Spruce (Picea abies L., Karst.). Plant Physiol. 1990, 94, 312–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Co, M.; Fagerlund, A.; Engman, L.; Sunnerheim, K.; Sjöberg, P.J.R.; Turner, C. Extraction of antioxidants from spruce (Picea abies) bark using eco-friendly solvents. Phytochem. Analysis 2012, 23, 1–11. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela-Raventós, R. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Matthes, A.; Schmitz-Eiberger, M. Polyphenol content and antioxidant capacity of apple fruit: Effect of cultivar and storage conditions. J. Appl. Bot. Food Qual. 2009, 82, 152–157. [Google Scholar]
- Hagerman, A.E. Tannin Chemistry. Available online: https://www.users.miamioh.edu/hagermae/ (accessed on 5 February 2018).
- DIN EN ISO 5267-1:2000. Pulps-Determination of Drainability-Part 1: Schopper-Riegler Method. Available online: https://standards.iteh.ai/catalog/standards/cen/c1a20a22-23c1-4c6f-b6bb-2441df91b1c5/en-iso-5267-1-2000 (accessed on 1 October 2020).
- DIN EN ISO 5269-2:2004. Pulps-Preparation of Laboratory Sheets for Physical Testing-Part 2: Rapid-Köthen Method. Available online: https://www.iso.org/obp/ui/#iso:std:iso:5269:-2:ed-3:v1:en (accessed on 1 October 2020).
- ISO 1924-2:2008. Paper and Board-Determination of Tensile Propertie-Part 2: Constant rate of Elongation Method (20 mm/min). Available online: https://www.iso.org/obp/ui/#iso:std:iso:1924:-2:ed-3:v1:en (accessed on 1 October 2020).
- Sharma, P.; Dubey, R.S. Drought Induces Oxidative Stress and Enhances the Activities of Antioxidant Enzymes in Growing Rice Seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Ramachandra Reddy, A.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Regier, M.; Mayer-Miebach, E.; Behsnilian, D.; Neff, E.; Schuchmann, H.P. Influences of Drying and Storage of Lycopene-Rich Carrots on the Carotenoid Content. Dry. Technol. 2005, 23, 989–998. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Plazola-Jacinto, C.P.; Hernández-Ortega, M.; Hernández-Navarro, M.D.; Villarreal, F.; Necoechea-Mondragón, H.; Ortiz-Moreno, A.; Ceballos-Reyes, G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. 2017, 41, 378–386. [Google Scholar] [CrossRef]
- Lewicki, P.P. Effect of pre-drying treatment, drying and rehydration on plant tissue properties: A review. Int. J. Food Prop. 1998, 1, 1–22. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Leikas, A.; Loponen, J.; Sinkkonen, J.; Ossipov, V.; Pihlaja, K. Reversed-phase HPLC-ESI/MS analysis of birch leaf proanthocyanidins after their acidic degradation in the presence of nucleophiles. Phytochem. Analysis 2007, 18, 378–386. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of cranberry extract powder as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e04777. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havelt, T.; Frase, J.N.; Pude, R.; Schmitz, M. Characterisation of Bioactive Ingredients in Extracts of Fresh and Dried Coniferous Trees for the Development of Sustainable Packaging Materials. Processes 2020, 8, 1366. https://doi.org/10.3390/pr8111366
Havelt T, Frase JN, Pude R, Schmitz M. Characterisation of Bioactive Ingredients in Extracts of Fresh and Dried Coniferous Trees for the Development of Sustainable Packaging Materials. Processes. 2020; 8(11):1366. https://doi.org/10.3390/pr8111366
Chicago/Turabian StyleHavelt, Thomas, Jan Niklas Frase, Ralf Pude, and Michaela Schmitz. 2020. "Characterisation of Bioactive Ingredients in Extracts of Fresh and Dried Coniferous Trees for the Development of Sustainable Packaging Materials" Processes 8, no. 11: 1366. https://doi.org/10.3390/pr8111366
APA StyleHavelt, T., Frase, J. N., Pude, R., & Schmitz, M. (2020). Characterisation of Bioactive Ingredients in Extracts of Fresh and Dried Coniferous Trees for the Development of Sustainable Packaging Materials. Processes, 8(11), 1366. https://doi.org/10.3390/pr8111366