Comparison of Dimethylformamide with Dimethylsulfoxide for Quality Improvement of Distillate Recovered from Waste Plastic Pyrolysis Oil
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Method
2.3. Material System and Conditions
3. Results and Discussion
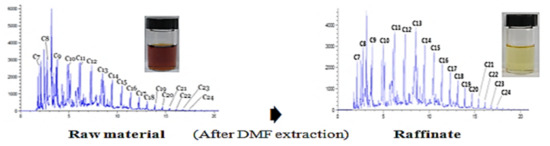
3.1. Gas Chromatogram of Raw Material (WPPO Distillate)
3.2. Equilibrium Extraction
4. Conclusions
Funding
Conflicts of Interest
References
- Syamsiro:, M.; Saptoadi, H.; Norsujianto, T.; Noviasri, P.; Cheng, S.; Alimuddin, Z.; Yoshikawa, K. Fuel oil production from municipal plastic wastes in sequential pyrolysis and catalytic reforming reactors. Energy Procedia 2014, 47, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Kalargaris, I.; Tian, G.; Gu, S. The utilization of oils produced from plastic waste at different pyrolysis temperatures in a DI diesel engine. Energy 2017, 131, 179–185. [Google Scholar] [CrossRef]
- Hartulistiyosoa, E.; Sigiroa, F.A.; Yuliantoa, M. Temperature distribution of the plastics pyrolysis process to produce fuel at 450 °C. Procedia Environ. Sci. 2015, 28, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Santaweesuk, C.; Janyalertadun, A. The production of fuel oil by conventional slow pyrolysis using plastic waste from a municipal landfill. IJESD 2017, 8, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Olufemi, A.S.; Olagboye, S.A. Thermal conversion of waste plastics into fuel oil. Int. J. Petrochem. Sci. Eng. 2017, 2, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Kalargaris, I.; Tian, G.; Gu, S. Experimental evaluation of a diesel engine fuelled by pyrolysis oils produced from low-density polyethylene and ethylene–vinyl acetate plastics. Fuel Process. Technol. 2017, 161, 125–131. [Google Scholar] [CrossRef]
- Frigo, S.; Seggiani, M.; Puccini, M.; Vitolo, S. Liquid fuel production from waste tyre pyrolysis and its utilization in a diesel engine. Fuel 2014, 116, 399–408. [Google Scholar] [CrossRef]
- Hamidi, N.; Tebyanian, F.; Massoudi, R.; Whitesides, L. Pyrolysis of household plastic wastes. Br. J. Appl. Sci. Technol. 2013, 3, 417–439. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Nahil, M.A.; Williams, P.T. Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils. J. Anal. Appl. Pyrolysis 2017, 124, 631–637. [Google Scholar] [CrossRef]
- Sharratt, P.N.; Lin, Y.H.; Garforth, A.A.; Dwyer, J. Investigation of the catalytic pyrolysis of high-density polyethylene over a HZSM-5 catalyst in a laboratory fluidized-bed reactor. Ind. Eng. Chem. Res. 1997, 36, 5118–5124. [Google Scholar] [CrossRef]
- Bagri, R.; Williams, P.T. Catalytic pyrolysis of polyethylene. J. Anal. Appl. Pyrolysis 2002, 63, 29–41. [Google Scholar] [CrossRef]
- Lee, K.H.; Shin, D.H. A comparative study of liquid product on non-catalytic and catalytic degradation of waste plastics using spent FCC catalyst. Korean J. Chem. Eng. 2006, 23, 209–215. [Google Scholar] [CrossRef]
- Koca, A.; Bilgesub, A.Y. Catalytic and thermal oxidative pyrolysis of LDPE in a continuous reactor system. J. Anal. Appl. Pyrolysis 2007, 78, 7–13. [Google Scholar] [CrossRef]
- Wiriyaumpaiwong, S.; Jamradloedluk, J. Fast pyrolysis of plastic wastes. Energy Procedia 2017, 138, 111–115. [Google Scholar] [CrossRef]
- Murugan, S.; Ramaswamy, M.C.; Nagarajan, G. The use of tyre pyrolysis oil in diesel engines. Waste Manag. 2008, 28, 2743–2749. [Google Scholar] [CrossRef]
- Kumar, R.; Mishra, M.K.; Singh, S.K.; Kumar, A. Experimental evaluation of waste plastic oil and its blends on a single cylinder diesel engine. J. Mech. Sci. Technol. 2016, 30, 4781–4789. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.C. Separation and recovery of dimethylnaphthalene isomers from light cycle oil by distillation-extraction combination. Sep. Sci. Technol. 2003, 38, 4095–4116. [Google Scholar] [CrossRef]
- Kim, S.J. Purification of 2,6-dimethylnaphthalene containing in light cycle oil by distillation-solvent extraction-solute crystallization combination. J. Ind. Eng. Chem. 2019, 79, 146–153. [Google Scholar] [CrossRef]
- Jiao, T.T.; Zhuang, X.L.; He, H.Y.; Li, C.S.; Chen, H.N.; Zhang, S.J. Separation of phenolic compounds from coal tar via liquid liquid extraction using amide compounds. Ind. Eng. Chem. Res. 2015, 54, 2573–2579. [Google Scholar] [CrossRef]
- Jiao, T.T.; Li, C.S.; Zhuang, X.L.; Cao, S.S.; Chen, H.N.; Zhang, S.J. The new liquid-liquid extraction method for separation of phenolic compounds from coal tar. Chem. Eng. J. 2015, 266, 148–155. [Google Scholar] [CrossRef]
- Kang, H.C.; Kim, S.J. Comparison of methanol with formamide on separation of nitrogen heterocyclic compounds from model coal tar fraction by batch cocurrent multistage equilibrium extraction. Polycycl. Aromat. Compd. 2016, 36, 745–757. [Google Scholar] [CrossRef]
- Kim, S.J.; Chun, Y.J. Separation of nitrogen heterocyclic compounds from model coal tar fraction by solvent extraction. Sep. Sci. Technol. 2005, 40, 2095–2109. [Google Scholar]
- Kim, S.J. Separation and purification of indole in model coal tar fraction of 9 compounds system. Polycycl. Aromat. Compd. 2019, 39, 60–72. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.C. Separation of valuable bicyclic aromatic components from light cycle oil by an emulsion liquid membrane. Sep. Sci. Technol. 2004, 39, 1093–1109. [Google Scholar] [CrossRef]
- Egashira, R.; Habaki, H.; Kawasaki, J. Decrease in aromatics content in motor gasoline by O/W/O emulsion liquid membrane process. J. Jpn. Pet. Inst. 1997, 40, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kang, H.C.; Kim, Y.S.; Jeong, H.J. Liquid membrane permeation of nitrogen heterocyclic compounds contained in model coal tar fraction. Bull. Korean Chem. Soc. 2010, 31, 1143–1148. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Goswami, A.N.; Rawat, B.S.; Krishina, R. Effect of surfactant type on the selectivity for separation of 1–methyl naphthalene and dodecane using liquid membranes. J. Membr. Sci. 1987, 42, 19–30. [Google Scholar] [CrossRef]
- Kang, H.C.; Shin, S.S.; Kim, D.H.; Kim, S.J. Recovery of paraffin components from pyrolysis oil fraction of waste plastic by batch cocurrent 4 stages equilibrium extraction. Appl. Chem. Eng. 2018, 29, 630–634. [Google Scholar]








| System | |
| Raw material | Distillate a of Waste Plastic Pyrolysis Oil (WPPO) |
| Solvent | (1) aqueous solution of dimethylformamide (DMF) |
| (2) aqueous solution of dimethylsulfoxide (DMSO) | |
| Experimental Conditions | |
| Liquid-liquid contacting time, t (h) | 12–72 |
| Mass fraction of water in solvent of initial state, yw,0 (–) | 0–0.06 |
| Mass ratio of solvent to raw material of initial state, (S/F)0 (–) | 1–10 |
| Operating temperature of initial state, T0 (°C) | 28 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J. Comparison of Dimethylformamide with Dimethylsulfoxide for Quality Improvement of Distillate Recovered from Waste Plastic Pyrolysis Oil. Processes 2020, 8, 1024. https://doi.org/10.3390/pr8091024
Kim SJ. Comparison of Dimethylformamide with Dimethylsulfoxide for Quality Improvement of Distillate Recovered from Waste Plastic Pyrolysis Oil. Processes. 2020; 8(9):1024. https://doi.org/10.3390/pr8091024
Chicago/Turabian StyleKim, Su Jin. 2020. "Comparison of Dimethylformamide with Dimethylsulfoxide for Quality Improvement of Distillate Recovered from Waste Plastic Pyrolysis Oil" Processes 8, no. 9: 1024. https://doi.org/10.3390/pr8091024
APA StyleKim, S. J. (2020). Comparison of Dimethylformamide with Dimethylsulfoxide for Quality Improvement of Distillate Recovered from Waste Plastic Pyrolysis Oil. Processes, 8(9), 1024. https://doi.org/10.3390/pr8091024