Centrifugal Filter-Assisted Block Freeze Crystallization Applied to Blueberry Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
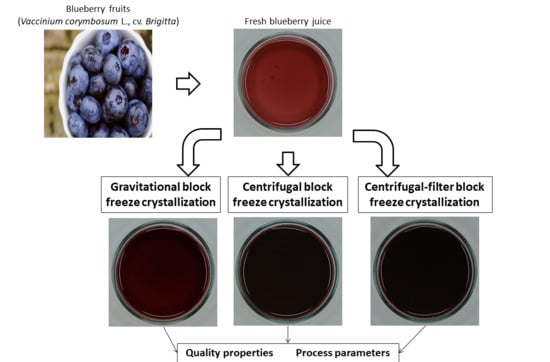
2.2. Raw Material and Juice Preparation
2.3. BFC Procedures
2.4. Determination of Physicochemical Properties
2.5. Determination of Total Phenolic Content (TPC), Total Anthocyanin Content (TAC), and Total Flavonoid Content (TFC)
2.6. Determination of Antioxidant Activity (AA)
2.7. Determination of the Process Parameters
2.7.1. Efficiency (η)
2.7.2. Solute Yield (Y)
2.7.3. Percentage of Concentrate (PC)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of Physicochemical Properties
3.2. Determination of Total Phenolic Content (TPC), Total Anthocyanin Content (TAC), and Total Flavonoid Content (TFC)
3.3. Determination of the Antioxidant Activity (AA)
3.4. Determination of Process Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef]
- de Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G.; Aadil, R.M. Comparison of high temperature-short time and sonication on selected parameters of strawberry juice during room temperature storage. J. Food Sci. Technol. 2020, 57, 1462–1468. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yang, C.Y. Ultrasound-assisted extraction of bioactive compounds and antioxidant capacity for the valorization of Elaeocarpus serratus L. leaves. Processes 2020, 8, 1218. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of polyphenolic profile and antioxidant activity of Pistacia lentiscus L. leaves and fruit extract obtained by optimized microwave-assisted extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Cheng, T.; Wang, S. Recent developments in applications of radio frequency heating for improving safety and quality of food grains and their products: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2622–2642. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Zeng, X.A.; Ren, E.F.; Xu, F.Y.; Li, J.; Wang, M.S.; Wang, R. Review of the application of pulsed electric fields (PEF) technology for food processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef] [PubMed]
- Salari, S.; Jafari, S.M. The influence of ohmic heating on degradation of food bioactive ingredients. Food Eng. Rev. 2020, 12, 191–208. [Google Scholar] [CrossRef]
- Munekata, P.E.; Domínguez, R.; Pateiro, M.; Lorenzo, J.M. Influence of plasma treatment on the polyphenols of food products—A review. Foods 2020, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.A.; Pintado, M.; Saraiva, J.A. High hydrostatic pressure-assisted extraction: A review on its effects on bioactive profile and biological activities of extracts. In Present and Future of High Pressure Processing, 1st ed.; Barba, F., Tonello-Samson, C., Puértolas, E., Lavilla, M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 317–328. [Google Scholar]
- Miyawaki, O.; Inakuma, T. Development of progressive freeze concentration and its application: A review. Food Bioproc Technol. 2021, 14, 39–51. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Tobar-Bolaños, G.; Casas-Forero, N.; Zúñiga, R.N.; Petzold, G. Quality attributes of cryoconcentrated calafate (Berberis microphylla) juice during refrigerated storage. Foods 2020, 9, 1314. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Guerra-Valle, M.; Astudillo-Lagos, M. Impact of block cryoconcentration on polyphenol retention in blueberry juice. Food Biosci. 2017, 20, 149–158. [Google Scholar] [CrossRef]
- Adorno, W.T.; Rezzadori, K.; Arend, G.D.; Chaves, V.C.; Reginatto, F.H.; di Luccio, M.; Petrus, J.C. Enhancement of phenolic compounds content and antioxidant activity of strawberry (Fragaria × ananassa) juice by block freeze concentration technology. Int. J. Food Sci. 2017, 52, 781–787. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; González, Y.; Petzold, G. Improvement of centrifugal cryoconcentration by ice recovery applied to orange juice. Chem. Eng. Technol. 2019, 42, 925–931. [Google Scholar] [CrossRef]
- Zielinski, A.A.; Zardo, D.M.; Alberti, A.; Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A. Effect of cryoconcentration process on phenolic compounds and antioxidant activity in apple juice. J. Sci. Food Agric. 2019, 99, 2786–2792. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Lazo-Mercado, V.; Gianelli, M.P.; Hernández, E.; Zúñiga, R.N.; Petzold, G. Influence of cryoconcentration on quality attributes of apple juice (Malus Domestica cv. Red Fuji). Appl. Sci. 2020, 10, 959. [Google Scholar] [CrossRef] [Green Version]
- Aider, M.; de Halleux, D.; Melnikova, I. Skim milk whey cryoconcentration and impact on the composition of the concentrated and ice fractions. Food Bioproc. Technol. 2009, 2, 80–88. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P. Emerging technologies available for the enhancement of bioactives concentration in functional beverages. In Biotechnological Progress and Beverage Consumption, 1st ed.; Grumezescu, A., Holban, A.M., Eds.; Academic Press: New York, NY, USA, 2020; pp. 39–69. [Google Scholar]
- Aider, M.; de Halleux, D. Passive and microwave-assisted thawing in maple sap cryoconcentration technology. J. Food Eng. 2008, 85, 65–72. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Andana, I.; Torres, N.; Cuevas, C. Retention of ascorbic acid and solid concentration via centrifugal freeze concentration of orange juice. J. Food Qual. 2017, 2017, 5214909. [Google Scholar] [CrossRef] [Green Version]
- Canella, M.H.M.; Dantas, A.; Blanco, M.; Raventós, M.; Hernandez, E.; Prudencio, E.S. Optimization of goat milk vacuum-assisted block freeze concentration using response surface methodology and NaCl addition influence. LWT-Food Sci. Technol. 2020, 124, 109133. [Google Scholar] [CrossRef]
- Orellana-Palma, P.A. External Forces Assisted Cryoconcentration to Improve the Concentration Process and the Quality of Fruit Juices. Ph.D. Thesis, Universidad del Bío-Bío, Chillán, Chile, May 2018. [Google Scholar]
- Orellana-Palma, P.; Takhar, P.; Petzold, G. Increasing the separation of block cryoconcentration through a novel centrifugal filter-based method. Sep. Sci. Technol. 2019, 54, 786–794. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Howard, L.R.; Brownmiller, C.R.; Prior, R.L. Influence of extrusion processing on procyanidin composition and total anthocyanin contents of blueberry pomace. J. Food Sci. 2009, 74, H52–H58. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.J.; Shin, Y. Assessment of physicochemical quality, antioxidant content and activity, and inhibition of cholinesterase between unripe and ripe blueberry fruit. Foods 2020, 9, 690. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Koca, I.; Karadeniz, B. Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea Region of Turkey. Sci. Hortic. 2009, 121, 447–450. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Cheng, K.; Peng, B.; Yuan, F. Volatile composition of eight blueberry cultivars and their relationship with sensory attributes. Flavour Fragr. J. 2020, 35, 443–453. [Google Scholar] [CrossRef]
- Madrera, R.R.; Valles, B.S.; Negrillo, A.C.; Fernández, J.F. Physicochemical characterization of blueberry (Vaccinium spp.) juices from 55 cultivars grown in Northern Spain. Acta Aliment. 2019, 48, 260–268. [Google Scholar] [CrossRef]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of anthocyanins content and profile throughout fruit development and ripening of highbush blueberry cultivars grown at two different altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Influence of block freeze concentration and evaporation on physicochemical properties, bioactive compounds and antioxidant activity in blueberry juice. Food Sci. Technol. 2020, 40, 387–394. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Zuñiga, R.N.; Takhar, P.S.; Gianelli, M.P.; Petzold, G. Effects of centrifugal block freeze crystallization on quality properties in pineapple juice. Chem. Eng. Technol. 2020, 43, 355–364. [Google Scholar] [CrossRef]
- Ding, Z.; Qin, F.G.; Yuan, J.; Huang, S.; Jiang, R.; Shao, Y. Concentration of apple juice with an intelligent freeze concentrator. J. Food Eng. 2019, 256, 61–72. [Google Scholar] [CrossRef]
- Meneses, D.L.; Ruiz, Y.; Hernandez, E.; Moreno, F.L. Multi-stage block freeze-concentration of green tea (Camellia sinensis) extract. J. Food Eng. 2021, 293, 110381. [Google Scholar] [CrossRef]
- Casas-Forero, N.; Moreno-Osorio, L.; Orellana-Palma, P.; Petzold, G. Effects of cryoconcentrate blueberry juice incorporation on gelatin gel: A rheological, textural and bioactive properties study. LWT-Food Sci. Technol. 2021, 138, 110674. [Google Scholar] [CrossRef]
- Petzold, G.; Moreno, J.; Lastra, P.; Rojas, K.; Orellana, P. Block freeze concentration assisted by centrifugation applied to blueberry and pineapple juices. Innov. Food Sci. Emerg. 2015, 30, 192–197. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Pierre, L.; Pensaben, J.M. Protection of polyphenols in blueberry juice by vacuum-assisted block freeze concentration. Food Chem. Toxicol. 2017, 109, 1093–1102. [Google Scholar] [CrossRef]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020, 307, 125549. [Google Scholar] [CrossRef]
- Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Comparative study of the structural properties, color, bioactive compounds content and antioxidant capacity of aerated gelatin gels enriched with cryoconcentrated blueberry juice during storage. Polymers 2020, 12, 2769. [Google Scholar] [CrossRef]
- González-Villagra, J.; Reyes-Díaz, M.; Alberdi, M.; Mora, M.L.; Ulloa-Inostroza, E.M.; Ribera-Fonseca, A.E. Impact of cold-storage and UV-C irradiation postharvest treatments on quality and antioxidant properties of fruits from blueberry cultivars grown in Southern Chile. J. Soil Sci. Plant Nutr. 2020, 20, 1751–1758. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.Y.; Jing, L.I.; Zhang, H.; Ye, L.I.; Farooq, S.; Bacha, S.A.S.; Wang, J. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Amran, N.A.; Rafee, N.H.M.; Samsuri, S. Retention of bioactive compound in the concentration of Centella asiatica extract through progressive freezing. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012175. [Google Scholar] [CrossRef]
- Bastías-Montes, J.M.; Martín, V.S.; Muñoz-Fariña, O.; Petzold-Maldonado, G.; Quevedo-León, R.; Wang, H.; Yang, Y.; Céspedes-Acuña, C.L. Cryoconcentration procedure for aqueous extracts of maqui fruits prepared by centrifugation and filtration from fruits harvested in different years from the same localities. J. Berry Res. 2019, 9, 377–394. [Google Scholar] [CrossRef]
- Azhar, A.N.; Panirselvam, M.; Amran, N.A.; Ruslan, M.S.; Samsuri, S. Retention of total phenolic content and antioxidant activity in the concentration of broccoli extract by progressive freeze concentration. Int. J. Food Eng. 2020, 16, 20190237. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Hybrid technologies: The future of energy efficient desalination—A review. Desalination 2020, 495, 114659. [Google Scholar] [CrossRef]
- Correa, L.J.; Ruiz, R.Y.; Moreno, F.L. Effect of falling-film freeze concentration on bioactive compounds in aqueous coffee extract. J. Food Process. Eng. 2018, 41, e12606. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Diaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in Southern Chile. J. Soil Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Barba, F.J.; Nemati, Z.; Shokofti, S.S.; Alizadeh, F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. J. Funct. Foods 2017, 38, 409–414. [Google Scholar] [CrossRef]
- Jaster, H.; Arend, G.D.; Rezzadori, K.; Chaves, V.C.; Reginatto, F.H.; Petrus, J.C.C. Enhancement of antioxidant activity and physicochemical properties of yogurt enriched with concentrated strawberry pulp obtained by block freeze concentration. Food Res. Int. 2018, 104, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Demoliner, F.; de Carvalho, L.T.; de Liz, G.R.; Prudêncio, E.S.; Ramos, J.C.; Bascuñan, V.L.A.F.; Vitali, L.; Block, J.M. Improving the nutritional and phytochemical compounds of a plant-based milk of sapucaia nut cake using block freeze concentration. Int. J. Food Sci. Technol. 2020, 55, 3031–3042. [Google Scholar] [CrossRef]
- Petzold, G.; Aguilera, J.M. Centrifugal freeze concentration. Innov. Food Sci. Emerg. Technol. 2013, 20, 253–258. [Google Scholar] [CrossRef]
- Vuist, J.E.; Boom, R.M.; Schutyser, M.A. Solute inclusion and freezing rate during progressive freeze concentration of sucrose and maltodextrin solutions. Dry. Technol. accepted. [CrossRef] [Green Version]
- Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Recovery of solutes from ice fractions in centrifugal block cryoconcentration applied to blueberry juice. Food Bioprocess. Technol. under review.



| Fresh Juice | GBFC | CBFC | CFBFC | |
|---|---|---|---|---|
| TSS (°Brix) (cryoconcentrated) | 13.8 ± 0.6 a | 23.4 ± 1.2 b | 29.5 ± 1.3 c | 35.9 ± 2.1 d |
| TSS (°Brix) (ice fraction) | 13.8 ± 0.6 a | 12.1 ± 0.8 b | 6.3 ± 0.2 c | 5.2 ± 0.4 d |
| pH | 3.1 ± 0.1 a | 2.8 ± 0.1 b | 2.5 ± 0.0 c | 2.3 ± 0.0 d |
| TTA (g MA/100 mL) | 0.7 ± 0.0 a | 1.0 ± 0.1 b | 1.4 ± 0.0 c | 1.9 ± 0.1 d |
| L* | 32.5 ± 1.4 a | 15.2 ± 1.5 b | 12.0 ± 1.1 c | 7.2 ± 2.5 d |
| a* | 7.3 ± 1.2 a | 4.0 ± 0.9 b | 2.9 ± 0.4 c | 1.2 ± 0.3 d |
| b* | 4.0 ± 0.0 a | 1.1 ± 0.0 b | 0.8 ± 0.2 c | 0.1 ± 0.1 d |
| ∆E* | - | 17.9 ± 1.3 a | 21.6 ± 1.7 b | 26.3 ± 1.0 c |
| Fresh Juice | GBFC | CBFC | CFBFC | |
|---|---|---|---|---|
| DPPH | 263.6 ± 10.3 a | 315.3 ± 22.9 b | 560.7 ± 60.3 c | 850.5 ± 32.7 d |
| FRAP | 312.2 ± 17.9 a | 498.3 ± 37.1 b | 855.8 ± 29.1 c | 1301.3 ± 55.9 d |
| ABTS | 574.6 ± 33.0 a | 739.6 ± 19.2 b | 1344.1 ± 27.9 c | 1768.2 ± 70.3 d |
| ORAC | 816.0 ± 22.7 a | 1091.4 ± 55.7 b | 1588.1 ± 43.7 c | 2741.1 ± 101.3 d |
| Process Parameters | GBFC | CBFC | CFBFC |
|---|---|---|---|
| Efficiency, % | 48.3 ± 5.4 a | 78.6 ± 2.9 b | 85.5 ± 1.4 c |
| Percentage of concentrate, % | 38.3 ± 3.1 a | 68.1 ± 4.0 b | 81.3 ± 2.0 c |
| Solute yield, kg/kg | 0.5 ± 0.1 a | 0.7 ± 0.0 b | 0.9 ± 0.0 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orellana-Palma, P.; Guerra-Valle, M.; Zúñiga, R.N. Centrifugal Filter-Assisted Block Freeze Crystallization Applied to Blueberry Juice. Processes 2021, 9, 421. https://doi.org/10.3390/pr9030421
Orellana-Palma P, Guerra-Valle M, Zúñiga RN. Centrifugal Filter-Assisted Block Freeze Crystallization Applied to Blueberry Juice. Processes. 2021; 9(3):421. https://doi.org/10.3390/pr9030421
Chicago/Turabian StyleOrellana-Palma, Patricio, María Guerra-Valle, and Rommy N. Zúñiga. 2021. "Centrifugal Filter-Assisted Block Freeze Crystallization Applied to Blueberry Juice" Processes 9, no. 3: 421. https://doi.org/10.3390/pr9030421
APA StyleOrellana-Palma, P., Guerra-Valle, M., & Zúñiga, R. N. (2021). Centrifugal Filter-Assisted Block Freeze Crystallization Applied to Blueberry Juice. Processes, 9(3), 421. https://doi.org/10.3390/pr9030421