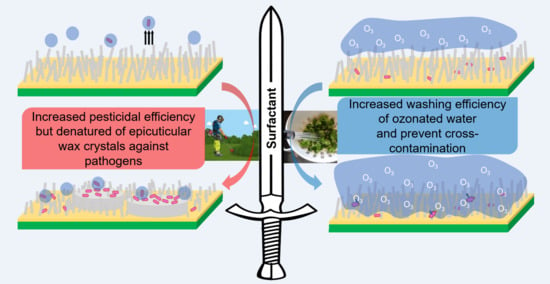
A Double-Edged Sword of Surfactant Effect on Hydrophobic Surface Broccoli Leaf as a Model Plant: Promotion of Pathogenic Microbial Contamination and Improvement to Disinfection Efficiency of Ozonated Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Near-Isogenic Broccoli: Greenhouse Production and Phenotype Description
2.2. Scanning Electron Microscope (SEM) Imaging of Leaf Surfaces
2.3. Leaf Disk Preparation and Spraying of Pesticidal Surfactant
2.4. Quantification and Composition of Epicuticular Wax
2.5. Contact Angle Measurement
2.6. S. Typhimurium Inoculation and Colony Number
2.7. Ozonated Water Preparation
2.8. Ozonated Water Treatment on Various Leaf Surfaces
2.9. Statistical Analysis
3. Results and Discussion
3.1. Images of Scanning Electron Microscope and Wax Quantification from Waxy and Glossy Near Isogenic Broccoli Lines
3.2. Pesticide Effects on S. Typhimurium Attachment to Waxy Leaves
3.3. Efficiency of Ozonated Water on Various Leaf Surfaces
3.4. Surfactant Effects on Leaf Surface Properties
3.5. Efficiency of Surfactant-Enhanced Ozonated Water on Waxy Leaves
3.6. The Potential and Disability of Surfactant for Decontamination on Waxy Leaves
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US Food and Drug Administration. Guidance for Industry: Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables; Center for Food Safety and Applied Nutrition: Washington, DC, USA, 1998. [Google Scholar]
- Buck, J.W.; Walcott, R.R.; Beuchat, L.R. Recent Trends in Microbiological Safety of Fruits and Vegetables. Plant Health Prog. 2003, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Warriner, K.; Huber, A.; Namvar, A.; Fan, W.; Dunfield, K. Chapter 4 Recent Advances in the Microbial Safety of Fresh Fruits and Vegetables. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2009; Volume 57, pp. 155–208. [Google Scholar]
- Scharff, R.L. Health-Related Costs from Foodborne Illness in the United States; Citeseer: University Park, PA, USA, 2010. [Google Scholar]
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mercanoglu Taban, B.; Halkman, A.K. Do leafy green vegetables and their ready-to-eat [RTE] salads carry a risk of foodborne pathogens? Anaerobe 2011, 17, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Klerks, M.M.; Franz, E.; van Gent-Pelzer, M.; Zijlstra, C.; van Bruggen, A.H.C. Differential interaction of Salmonella enterica serovars with lettuce cultivars and plant-microbe factors influencing the colonization efficiency. Isme J. 2007, 1, 620–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA anel on Biological Hazards. Scientific Opinion on the risk posed by pathogens in food of non-animal origin. Part 1 (outbreak data analysis and risk ranking of food/pathogen combinations). Efsa J. 2013, 11, 3025. [Google Scholar] [CrossRef] [Green Version]
- Beuchat, L.R. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 2002, 4, 413–423. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Ryu, J.H. Produce handling and processing practices. Emerg. Infect. Dis. 1997, 3, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Navratil, S.; Gregory, A.; Bauer, A.; Srinath, I.; Szonyi, B.; Nightingale, K.; Anciso, J.; Jun, M.; Han, D.; et al. Multifactorial Effects of Ambient Temperature, Precipitation, Farm Management, and Environmental Factors Determine the Level of Generic Escherichia coli Contamination on Preharvested Spinach. Appl. Environ. Microbiol. 2015, 81, 2635–2650. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Jang, H.; Matthews, K.R. Effect of the food production chain from farm practices to vegetable processing on outbreak incidence. Microb. Biotechnol. 2014, 7, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Bargel, H.; Koch, K.; Cerman, Z.; Neinhuis, C. Evans Review No. 3: Structurefunction relationships of the plant cuticle and cuticular waxes a smart material? Funct. Plant Biol. 2006, 33, 893–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, K.-M.; Chiu, Y.-C.; Shen, C.; Jenks, M. Leaf cuticular waxes of lettuce are associated with reduced attachment of the foodborne pathogen Salmonella spp. at harvest and after postharvest storage. LWT 2020, 117, 108657. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Shen, C.; Farnham, M.W.; Ku, K.-M. Three-dimensional epicuticular wax on plant surface reduces attachment and survival rate of Salmonella during storage. Postharvest Biol. Technol. 2020, 166, 111197. [Google Scholar] [CrossRef]
- Patel, J.; Sharma, M. Differences in attachment of Salmonella enterica serovars to cabbage and lettuce leaves. Int. J. Food Microbiol. 2010, 139, 41–47. [Google Scholar] [CrossRef]
- Holvoet, K.; Jacxsens, L.; Sampers, I.; Uyttendaele, M. Insight into the Prevalence and Distribution of Microbial Contamination To Evaluate Water Management in the Fresh Produce Processing Industry. J. Food Prot. 2012, 75, 671–681. [Google Scholar] [CrossRef]
- Banach, J.L.; Sampers, I.; Van Haute, S.; der Fels-Klerx, V. Effect of disinfectants on preventing the cross-contamination of pathogens in fresh produce washing water. Int. J. Environ. Res. Public Health 2015, 12, 8658–8677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Nabulsi, A.A.; Osaili, T.M.; Obaidat, H.M.; Shaker, R.R.; Awaisheh, S.S.; Holley, R.A. Inactivation of Stressed Escherichia coli O157:H7 Cells on the Surfaces of Rocket Salad Leaves by Chlorine and Peroxyacetic Acid. J. Food Prot. 2014, 77, 32–39. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Allende, A.; Selma, M.V.; Gil, M.I. Prevention of Escherichia coli cross-contamination by different commercial sanitizers during washing of fresh-cut lettuce. Int. J. Food Microbiol. 2009, 133, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Yuk, H.-G.; Yoo, M.-Y.; Yoon, J.-W.; Moon, K.-D.; Marshall, D.L.; Oh, D.-H. Effect of Combined Ozone and Organic Acid Treatment for Control of Escherichia coli O157:H7 and Listeria monocytogenes on Lettuce. J. Food Sci. 2006, 71, M83–M87. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Sun, Y.; Li, C.; Li, Y.; Zhang, Q.; Wu, Z. Reduction of Escherichia coli O157:H7 and naturally present microbes on fresh-cut lettuce using lactic acid and aqueous ozone. RSC Adv. 2019, 9, 22636–22643. [Google Scholar] [CrossRef] [Green Version]
- Papachristodoulou, M.; Koukounaras, A.; Siomos, A.S.; Liakou, A.; Gerasopoulos, D. The effects of ozonated water on the microbial counts and the shelf life attributes of fresh-cut spinach. J. Food Process. Preserv. 2018, 42, e13404. [Google Scholar] [CrossRef]
- Xu, W.; Wu, C. Different Efficiency of Ozonated Water Washing to Inactivate Salmonella enterica Typhimurium on Green Onions, Grape Tomatoes, and Green Leaf Lettuces. J. Food Sci. 2014, 79, M378–M383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Z.; Yu, Z.; Gao, X. Preservation of fresh-cut celery by treatment of ozonated water. Food Control 2005, 16, 279–283. [Google Scholar] [CrossRef]
- Farnham, M.W. Glossy and Nonglossy Near-isogenic Lines USVL115-GL, USVL115-NG, USVL188-GL, and USVL188-NG of Broccoli. HortScience 2010, 45, 660–662. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Ku, K.-M.; Palma-Salgado, S.P.; Storm, A.P.; Feng, H.; Juvik, J.A.; Nguyen, T.H. Influence of Epicuticular Physicochemical Properties on Porcine Rotavirus Adsorption to 24 Leafy Green Vegetables and Tomatoes. PLoS ONE 2015, 10, e0132841. [Google Scholar] [CrossRef] [Green Version]
- Holloway, P.J.; Brown, G.A.; Baker, E.A.; Macey, M.J.K. Chemical composition and ultrastructure of the epicuticular wax in three lines of Brassica napus (L). Chem. Phys. Lipids 1977, 19, 114–127. [Google Scholar] [CrossRef]
- Wen, M.; Jetter, R. Composition of secondary alcohols, ketones, alkanediols, and ketols in Arabidopsis thaliana cuticular waxes. J. Exp. Bot. 2009, 60, 1811–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, K.; Matute, C.M.; Hassan, A.N.; Frank, J.F. Comparison of the Attachment of Escherichia coli O157:H7, Listeria monocytogenes, Salmonella Typhimurium, and Pseudomonas fluorescens to Lettuce Leaves. J. Food Prot. 2000, 63, 1433–1437. [Google Scholar] [CrossRef]
- Hunter, P.J.; Shaw, R.K.; Berger, C.N.; Frankel, G.; Pink, D.; Hand, P. Older leaves of lettuce (Lactuca spp.) support higher levels of Salmonella enterica ser. Senftenberg attachment and show greater variation between plant accessions than do younger leaves. Fems Microbiol. Lett. 2015, 362, fnv077. [Google Scholar] [CrossRef] [Green Version]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Ölmez, H. Effectiveness of organic acid, ozonated water and chlorine dippings on microbial reduction and storage quality of fresh-cut iceberg lettuce. J. Sci. Food Agric. 2007, 87, 2609–2616. [Google Scholar] [CrossRef]
- Hassan, A.N.; Frank, J.F. Influence of surfactant hydrophobicity on the detachment of Escherichia coli O157:H7 from lettuce. Int. J. Food Microbiol. 2003, 87, 145–152. [Google Scholar] [CrossRef]
- Brandl, M.T.; Huynh, S. Effect of the Surfactant Tween 80 on the Detachment and Dispersal of Salmonella enterica Serovar Thompson Single Cells and Aggregates from Cilantro Leaves as Revealed by Image Analysis. Appl. Environ. Microbiol. 2014, 80, 5037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Nitin, N. Enhanced removal of Escherichia coli O157:H7 and Listeria innocua from fresh lettuce leaves using surfactants during simulated washing. Food Control 2017, 79, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Tian, Y.; Salvi, D.; Karwe, M.; Nitin, N. Influence of Exposure Time, Shear Stress, and Surfactants on Detachment of Escherichia coli O157:H7 from Fresh Lettuce Leaf Surfaces During Washing Process. Food Bioprocess Technol. 2018, 11, 621–633. [Google Scholar] [CrossRef]
- Koch, K.; Hartmann, K.D.; Schreiber, L.; Barthlott, W.; Neinhuis, C. Influences of air humidity during the cultivation of plants on wax chemical composition, morphology and leaf surface wettability. Environ. Exp. Bot. 2006, 56, 1–9. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.J.; Kim, M.H.; Ku, K.-M. A Double-Edged Sword of Surfactant Effect on Hydrophobic Surface Broccoli Leaf as a Model Plant: Promotion of Pathogenic Microbial Contamination and Improvement to Disinfection Efficiency of Ozonated Water. Processes 2021, 9, 679. https://doi.org/10.3390/pr9040679
Song HJ, Kim MH, Ku K-M. A Double-Edged Sword of Surfactant Effect on Hydrophobic Surface Broccoli Leaf as a Model Plant: Promotion of Pathogenic Microbial Contamination and Improvement to Disinfection Efficiency of Ozonated Water. Processes. 2021; 9(4):679. https://doi.org/10.3390/pr9040679
Chicago/Turabian StyleSong, Hyun Jong, Min Hwan Kim, and Kang-Mo Ku. 2021. "A Double-Edged Sword of Surfactant Effect on Hydrophobic Surface Broccoli Leaf as a Model Plant: Promotion of Pathogenic Microbial Contamination and Improvement to Disinfection Efficiency of Ozonated Water" Processes 9, no. 4: 679. https://doi.org/10.3390/pr9040679
APA StyleSong, H. J., Kim, M. H., & Ku, K. -M. (2021). A Double-Edged Sword of Surfactant Effect on Hydrophobic Surface Broccoli Leaf as a Model Plant: Promotion of Pathogenic Microbial Contamination and Improvement to Disinfection Efficiency of Ozonated Water. Processes, 9(4), 679. https://doi.org/10.3390/pr9040679