Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. The Impact of Low Temperatures: 1000 K and 1500 K
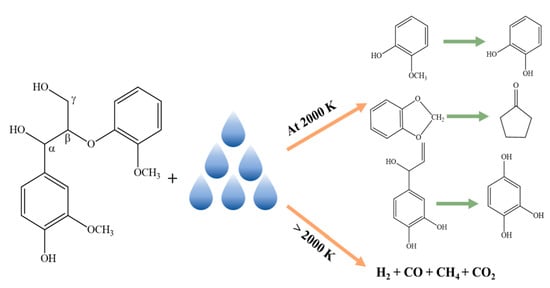
3.2. At Temperature 2000 K
3.3. 2500 K and Higher Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Lebaka, V.R. Potential Bioresources as Future Sources of Biofuels Production: An Overview. Biofuel Technol. 2013, 223–258. [Google Scholar] [CrossRef]
- Commodities 2021: Climate Change Policy Targets to Stimulate EU Biodiesel Consumption|S&P Global Platts. Available online: https://www.spglobal.com/platts/en/market-insights/latest-news/agriculture/122320-commodities-2021-climate-change-policy-targets-to-stimulate-eu-biodiesel-consumption (accessed on 24 March 2021).
- Sandak, A.; Sandak, J. Utilization of FT-NIR for Proper Biomass Conversion. In Proceedings of the NIR2013-A1—Agriculture, Environment, La Grande-Motte, France, 2 June 2013. [Google Scholar]
- Anukam, A.; Berghel, J. Biomass Pretreatment and Characterization: A Review. Biomass 2020. [Google Scholar] [CrossRef]
- Lee, S.; Shah, Y.T. Biofuels and Bioenergy: Processes and Technologies; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4200-8955-4. [Google Scholar]
- Poletto, M. Lignin: Trends and Applications; BoD—Books on Demand: Norderstedt, Germany, 2018; ISBN 978-953-51-3901-0. [Google Scholar]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Holladay, J.E.; Bozell, J.J.; White, J.F.; Johnson, D. Results of Screening for Potential Candidates from Biorefinery Lignin. In Top Value Added Candidates Biomass; U.S. Department of Energy: Washington, DC, USA, 2007; pp. 53–55. [Google Scholar]
- Sinha, S.; Jhalani, A.; Ravi, M.R.; Ray, A. Modelling of Pyrolysis in Wood: A Review. SESI J. 2000, 10, 41–62. [Google Scholar]
- Guan, Q.; Mao, T.; Zhang, Q.; Miao, R.; Ning, P.; Gu, J.; Tian, S.; Chen, Q.; Chai, X.-S. Catalytic Gasification of Lignin with Ni/Al2O3–SiO2 in Sub/Supercritical Water. J. Supercrit. Fluids 2014, 95, 413–421. [Google Scholar] [CrossRef]
- Barati, M.; Babatabar, M.; Tavasoli, A.; Dalai, A.K.; Das, U. Hydrogen Production via Supercritical Water Gasification of Bagasse Using Unpromoted and Zinc Promoted Ru/γ-Al2O3 Nanocatalysts. Fuel Process. Technol. 2014, 123, 140–148. [Google Scholar] [CrossRef]
- Safari, F.; Tavasoli, A.; Ataei, A.; Choi, J.-K. Hydrogen and Syngas Production from Gasification of Lignocellulosic Biomass in Supercritical Water Media. Int. J. Recycl. Org. Waste Agric. 2015, 4, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical Water Gasification of Biomass for Hydrogen Production. Int. J. Hydrog. Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Guo, L.J.; Lu, Y.J.; Zhang, X.M.; Ji, C.M.; Guan, Y.; Pei, A.X. Hydrogen Production by Biomass Gasification in Supercritical Water: A Systematic Experimental and Analytical Study. Catal. Today 2007, 129, 275–286. [Google Scholar] [CrossRef]
- Saxena, R.C.; Seal, D.; Kumar, S.; Goyal, H.B. Thermo-Chemical Routes for Hydrogen Rich Gas from Biomass: A Review. Renew. Sustain. Energy Rev. 2008, 12, 1909–1927. [Google Scholar] [CrossRef]
- Calzavara, Y.; Joussot-Dubien, C.; Boissonnet, G.; Sarrade, S. Evaluation of Biomass Gasification in Supercritical Water Process for Hydrogen Production. Energy Convers. Manag. 2005, 46, 615–631. [Google Scholar] [CrossRef]
- Resende, F.L.P.; Savage, P.E. Kinetic Model for Noncatalytic Supercritical Water Gasification of Cellulose and Lignin. AIChE J. 2010, 56, 2412–2420. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Gorman, D.J.; Tiwari, K.; Rasmussen, E.G.; Kramlich, J.C.; Reinhall, P.G.; Novosselov, I.V. Supercritical Water Gasification: Practical Design Strategies and Operational Challenges for Lab-Scale, Continuous Flow Reactors. Heliyon 2019, 5, e01269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Oshima, Y.; Matsumura, Y. Gasification of Biomass Model Compounds and Real Biomass in Supercritical Water. Biomass Bioenergy 2004, 26, 71–78. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsumura, Y. Gasification of Cellulose, Xylan, and Lignin Mixtures in Supercritical Water. Ind. Eng. Chem. Res. 2001, 40, 5469–5474. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Wu, Y.; Guo, L.; Su, X. Molecular Dynamic Investigation on Hydrogen Production by Polycyclic Aromatic Hydrocarbon Gasification in Supercritical Water. Int. J. Hydrog. Energy 2016, 41, 3837–3843. [Google Scholar] [CrossRef]
- Jin, H.; Wu, Y.; Zhu, C.; Guo, L.; Huang, J. Molecular Dynamic Investigation on Hydrogen Production by Furfural Gasification in Supercritical Water. Int. J. Hydrog. Energy 2016, 41, 16064–16069. [Google Scholar] [CrossRef]
- Jin, H.; Chen, B.; Zhao, X.; Cao, C. Molecular Dynamic Simulation of Hydrogen Production by Catalytic Gasification of Key Intermediates of Biomass in Supercritical Water. J. Energy Resour. Technol. 2017, 140. [Google Scholar] [CrossRef]
- Rismiller, S.C.; Groves, M.M.; Meng, M.; Dong, Y.; Lin, J. Water Assisted Liquefaction of Lignocellulose Biomass by ReaxFF Based Molecular Dynamic Simulations. Fuel 2018, 215, 835–843. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Z.; Li, X.; Qiao, X.; Song, W.; Guo, L. Initial Reaction Mechanisms of Cellulose Pyrolysis Revealed by ReaxFF Molecular Dynamics. Fuel 2016, 177, 130–141. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Qiao, X.; Zheng, M.; Guo, L.; Song, W.; Lin, W. Initial Mechanisms for an Overall Behavior of Lignin Pyrolysis through Large-Scale ReaxFF Molecular Dynamics Simulations. Energy Fuels 2016, 30, 3140–3150. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Guo, L.; Guo, X. Reaction Mechanisms in Pyrolysis of Hardwood, Softwood, and Kraft Lignin Revealed by ReaxFF MD Simulations. Energy Fuels 2019, 33, 11210–11225. [Google Scholar] [CrossRef]
- Li, H.; Xu, B.; Jin, H.; Luo, K.; Fan, J. Molecular Dynamics Investigation on the Lignin Gasification in Supercritical Water. Fuel Process. Technol. 2019, 192, 203–209. [Google Scholar] [CrossRef]
- Han, Y.; Chen, F.; Ma, T.; Gong, H.; Al-Shwafy, K.W.A.; Li, W.; Zhang, J.; Zhang, M. Size Effect of a Ni Nanocatalyst on Supercritical Water Gasification of Lignin by Reactive Molecular Dynamics Simulations. Ind. Eng. Chem. Res. 2019, 58, 23014–23024. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chu, J.; He, M.; Li, Q.; Zhang, Y. Understanding Lignin Gasification in Supercritical Water Using Reactive Molecular Dynamics Simulations. Renew. Energy 2020, 161, 858–866. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Gordobil, O.; Diaz, R.H.; Sandak, A.; Sandak, J. Fractionation of Lignin Using Organic Solvents: A Combined Experimental and Theoretical Study. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General Atomic and Molecular Electronic Structure System. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Dong, K.; Fan, M.; Zhang, S. A DFT Study on Lignin Dissolution in Imidazolium-Based Ionic Liquids. RSC Adv. 2017, 7, 12670–12681. [Google Scholar] [CrossRef] [Green Version]
- Ju, Z.; Xiao, W.; Yao, X.; Tan, X.; Simmons, B.A.; Sale, K.L.; Sun, N. Theoretical Study on the Microscopic Mechanism of Lignin Solubilization in Keggin-Type Polyoxometalate Ionic Liquids. Phys. Chem. Chem. Phys. 2020, 22, 2878–2886. [Google Scholar] [CrossRef]
- Melián-Rodríguez, M.; Saravanamurugan, S.; Meier, S.; Kegnæs, S.; Riisager, A. Ru-Catalyzed Oxidative Cleavage of Guaiacyl Glycerol-β-Guaiacyl Ether-a Representative β-O-4 Lignin Model Compound. Catalysts 2019, 9, 832. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Han, Z.; Fu, L.; Liu, C.; Zhang, D. Cleavage of the β–O–4 Bond in a Lignin Model Compound Using the Acidic Ionic Liquid 1-H-3-Methylimidazolium Chloride as Catalyst: A DFT Mechanistic Study. J. Mol. Model. 2018, 24, 322. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhong, W.; Wu, K.; Wei, G.; Hu, Z.; Zheng, W.; Ruan, H.; Zhang, H.; Xiao, R. Catalytic Pyrolysis Mechanism of β-O-4 Type of Lignin Dimer: The Role of H Proton. Energy Fuels 2021, 35, 575–582. [Google Scholar] [CrossRef]
- Morales, G.; Iglesias, J.; Melero, J.A. Sustainable Catalytic Conversion of Biomass for the Production of Biofuels and Bioproducts. Catalysts 2020, 10, 581. [Google Scholar] [CrossRef]
- Drage, T.C.; Vane, C.H.; Abbott, G.D. The Closed System Pyrolysis of β-O-4 Lignin Substructure Model Compounds. Org. Geochem. 2002, 33, 1523–1531. [Google Scholar] [CrossRef]
- He, T.; Zhang, Y.; Zhu, Y.; Wen, W.; Pan, Y.; Wu, J.; Wu, J. Pyrolysis Mechanism Study of Lignin Model Compounds by Synchrotron Vacuum Ultraviolet Photoionization Mass Spectrometry. Energy Fuels 2016, 30, 2204–2208. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, B.; Xie, W.; Jiang, X.; Liu, J.; Lu, Q. Recent Progress in Quantum Chemistry Modeling on the Pyrolysis Mechanisms of Lignocellulosic Biomass. Energy Fuels 2020, 34, 10384–10440. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Chenoweth, K.; van Duin, A.C.T.; Goddard, W.A. ReaxFF Reactive Force Field for Molecular Dynamics Simulations of Hydrocarbon Oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Cao, L.; Chin, C.-H.; Ren, H.; Zhang, J.Z.H.; Zhu, T. ReacNetGenerator: An Automatic Reaction Network Generator for Reactive Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2020, 22, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H. Lignin Pyrolysis Reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef] [Green Version]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance Material Review on 1,3-Benzodioxole-5-Propanol, α-Methyl-, 5-Acetate. Food Chem. Toxicol. 2012, 50, S330–S332. [Google Scholar] [CrossRef]
- Pingali, S.R.K.; Jursic, B.S. Microwave-Assisted Synthesis of 1,3-Benzodioxole Derivatives from Catechol and Ketones or Aldehydes. Tetrahedron Lett. 2011, 52, 4371–4374. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, L.; Jin, H.; Ou, Z.; Wei, W.; Huang, J. Gasification of Guaiacol in Supercritical Water: Detailed Reaction Pathway and Mechanisms. Int. J. Hydrog. Energy 2018, 43, 14078–14086. [Google Scholar] [CrossRef]
- Cao, C.; Xie, Y.; Li, L.; Wei, W.; Jin, H.; Wang, S.; Li, W. Supercritical Water Gasification of Lignin and Cellulose Catalyzed with Co-Precipitated CeO2–ZrO2. Energy Fuels 2021. [Google Scholar] [CrossRef]
- Miliotti, E.; Dell’Orco, S.; Lotti, G.; Rizzo, A.M.; Rosi, L.; Chiaramonti, D. Lignocellulosic Ethanol Biorefinery: Valorization of Lignin-Rich Stream through Hydrothermal Liquefaction. Energies 2019, 12, 723. [Google Scholar] [CrossRef] [Green Version]
- Okuda, K.; Umetsu, M.; Takami, S.; Adschiri, T. Disassembly of Lignin and Chemical Recovery—Rapid Depolymerization of Lignin without Char Formation in Water–Phenol Mixtures. Fuel Process. Technol. 2004, 85, 803–813. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Labidi, J. Improving Base Catalyzed Lignin Depolymerization by Avoiding Lignin Repolymerization. Fuel 2014, 116, 617–624. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of Current and Future Softwood Kraft Lignin Process Chemistry. Ind. Crop. Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]






| H2O | → | H˙ | + | HO˙ | ||
| 2 H˙ | → | H2 | ||||
| HCOOH | → | HCOO˙ | + | H˙ | ||
| HCOO˙ | → | CO2 | + | H˙ | ||
| HCOO˙ | → | CO | + | HO˙ | ||
| HCOO˙ | + | H˙ | → | CO2 | + | H2 |
| HCOO˙ | + | OH˙ | → | CO2 | + | H2O |
| H2O2 | → | HOO˙ | + | H˙ | ||
| H2O2 | → | O2 | + | H2 | ||
| H2O2 | → | 2 HO˙ | ||||
| CH4 | + | OH˙ | → | CH3˙ | + | H2O |
| CH3˙ | + | H˙ | → | CH2˙˙ | + | H2 |
| CH2˙˙ | → | C2H4 | ||||
| CH3˙ | + | H2 | → | CH4 | + | H˙ |
| CH3˙ | + | H2O | → | CH3OH | + | H˙ |
| CH3OH | + | HO˙ | → | [CH2OH] ˙ | + | H2O |
| CH3OH | + | H˙ | → | [CH2OH] ˙ | + | H2 |
| [CH2OH] ˙ | + | H2 | → | CH4 | + | HO˙ |
| [CH2OH] ˙ | + | H2O | → | CH3O˙ | + | HO˙ |
| CH3O˙ | + | H˙ | → | CO | + | 2H2 |
| HO-C≡C-OH | + | 4 H˙ | → | ˙C≡C˙ | + | 2 H2O |
| ˙C≡C˙ | + | 2 H˙ | → | HC≡CH | ||
| HC≡CH | + | 2 H˙ | → | H2C=CH2 | ||
| H2C=CH2 | + | 4 H˙ | → | 2 CH4 | ||
| CH4 | + | H2O | → | CO | + | 6 H2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponnuchamy, V.; Sandak, J.; Sandak, A. Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations. Processes 2021, 9, 714. https://doi.org/10.3390/pr9040714
Ponnuchamy V, Sandak J, Sandak A. Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations. Processes. 2021; 9(4):714. https://doi.org/10.3390/pr9040714
Chicago/Turabian StylePonnuchamy, Veerapandian, Jakub Sandak, and Anna Sandak. 2021. "Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations" Processes 9, no. 4: 714. https://doi.org/10.3390/pr9040714
APA StylePonnuchamy, V., Sandak, J., & Sandak, A. (2021). Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations. Processes, 9(4), 714. https://doi.org/10.3390/pr9040714