Facile Preparation of Phenyboronic-Acid-Functionalized Fe3O4 Magnetic Nanoparticles for the Selective Adsorption of Ortho-Dihydroxy-Containing Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Material
2.2. Synthesis of Fe3O4@PBA Nanoparticles
2.2.1. Synthesis of Fe3O4 Nanoparticles
2.2.2. Synthesis of Fe3O4@MPTES
2.2.3. Synthesis of Fe3O4@MPS
2.2.4. Phenyboronic-Acid-Functionalized Fe3O4@MPS and Fe3O4@MPTES
2.3. Binding Experiments
2.4. Selectivity Experiments
3. Results and Discussion
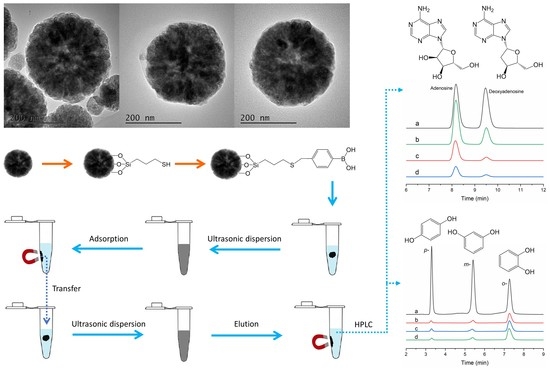
3.1. Characterization of Fe3O4@PBA Nanoparticles
3.2. Binding Properties of Fe3O4@PBA Nanoparticles
3.2.1. Thermodynamics of Adsorption
3.2.2. Kinetics of Adsorption
3.3. Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prucek, R.; Tucek, J.; Kilianova, M.; Panacek, A.; Kvitek, L.; Filip, J.; Kolar, M.; Tomankova, K.; Zboril, R. The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles. Biomaterials 2011, 32, 4704–4713. [Google Scholar] [CrossRef] [PubMed]
- Erathodiyil, N.; Ying, J.Y. Functionalization of inorganic nanoparticles for bioimaging applications. Acc. Chem. Res. 2011, 44, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gao, J.H.; Ai, H.; Chen, X.Y. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Babamiri, B.; Salimi, A.; Hallaj, R. Switchable electrochemiluminescence aptasensor coupled with resonance energy transfer for selective attomolar detection of Hg2+ via CdTe@CdS/dendrimer probe and Au nanoparticle quencher. Biosens. Bioelectron. 2018, 102, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Moazzen, M.; Ahmadkhaniha, R.; Gorji, M.E.; Yunesian, M.; Rastkari, N. Magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes for the determination of polycyclic aromatic hydrocarbons in grilled meat samples. Talanta 2013, 115, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Facchi, D.P.; Cazetta, A.L.; Canesin, E.A.; Almeida, V.C.; Bonafé, E.G.; Kipper, M.J.; Martins, A.F. New magnetic chitosan/alginate/Fe3O4@SiO2 hydrogel composites applied for removal of Pb(II) ions from aqueous systems. Chem. Eng. J. 2018, 337, 595–608. [Google Scholar] [CrossRef]
- Fan, R.; Min, H.; Hong, X.; Yi, Q.; Liu, W.; Zhang, Q.; Luo, Z. Plant tannin immobilized Fe3O4@SiO2 microspheres: A novel and green magnetic bio-sorbent with superior adsorption capacities for gold and palladium. J. Hazard. Mater. 2019, 364, 780–790. [Google Scholar] [CrossRef]
- Cai, Y.R.; Pan, H.H.; Xu, X.R.; Hu, Q.H.; Li, L.; Tang, R.K. Ultrasonic controlled morphology transformation of hollow calcium phosphate nanospheres: A smart and biocompatible drug release system. Chem. Mater. 2007, 19, 3081–3083. [Google Scholar] [CrossRef]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Niu, N.; Gao, X.; Han, F.; Chen, Z.; Li, S.; Li, J. A new drug carrier with oxygen generation function for modulating tumor hypoxia microenvironment in cancer chemotherapy. Colloids Surf. B. Biointerfaces 2019, 173, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Justin, R.; Tao, K.; Román, S.; Chen, D.; Xu, Y.; Geng, X.; Ross, I.M.; Grant, R.T.; Pearson, A.; Zhou, G.; et al. Photoluminescent and superparamagnetic reduced graphene oxide-iron oxide quantum dots for dual-modality imaging, drug delivery and photothermal therapy. Carbon 2016, 97, 54–70. [Google Scholar] [CrossRef] [Green Version]
- Sonvico, F.; Dubernet, C.; Colombo, P.; Couvreur, P. Metallic colloid nanotechnology, applications in diagnosis and therapeutics. Curr. Pharm. Des. 2005, 11, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, S. Monodisperse magnetic nanoparticles for biomedical applications. Polym. Int. 2007, 56, 821–826. [Google Scholar] [CrossRef]
- Villalonga, M.L.; Borisova, B.; Arenas, C.B.; Villalonga, A.; Arévalo-Villena, M.; Sánchez, A.; Pingarrón, J.M.; Briones-Pérez, A.; Villalonga, R. Disposable electrochemical biosensors for Brettanomyces bruxellensis and total yeast content in wine based on core-shell magnetic nanoparticles. Sensor. Actuat. B Chem. 2019, 279, 15–21. [Google Scholar] [CrossRef]
- Gu, H.; Ho, P.L.; Tsang, K.W.; Wang, L.; Xu, B. Using biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram-positive bacteria at ultralow concentration. J. Am. Chem. Soc. 2003, 125, 15702–15703. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wu, L.; Duan, J.; Yin, H.; Ai, S. Ultrasensitive electrochemiluminescence immunosensor for 5-hydroxymethylcytosine detection based on Fe3O4@SiO2 nanoparticles and PAMAM dendrimers. Biosens. Bioelectron. 2018, 99, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, H. Synthesis and applications of boronate affinity materials: From class selectivity to biomimetic specificity. Acc. Chem. Res. 2017, 50, 2185–2193. [Google Scholar] [CrossRef] [Green Version]
- Brooks, W.L.; Sumerlin, B.S. Synthesis and applications of boronic acid-containing polymers: From materials to medicine. Chem. Rev. 2016, 116, 1375–1397. [Google Scholar] [CrossRef]
- Li, D.J.; Chen, Y.; Liu, Z. Boronate affinity materials for separation and molecular recognition: Structure, properties and applications. Chem. Soc. Rev. 2015, 44, 8097–8123. [Google Scholar] [CrossRef]
- Nishiyabu, R.; Kubo, Y.; James, T.D.; Fossey, J.S. Boronic acid building blocks: Tools for sensing and separation. Chem. Commun. 2011, 47, 1106–1123. [Google Scholar] [CrossRef]
- Xing, R.; Wang, S.; Bie, Z.; He, H.; Liu, Z. Preparation of molecularly imprinted polymers specific to glycoproteins, glycans and monosaccharides via boronate affinity controllable-oriented surface imprinting. Nat. Protoc. 2017, 12, 964–987. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Huang, J.; Cong, J.J.; Wei, W.; Liu, X.Y. Double recognition and selective extraction of glycoprotein based on the molecular imprinted graphene oxide and boronate affinity. ACS Appl. Mater. Interfaces 2017, 9, 7735–7744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Li, P.; Xiao, H.B.; Wang, H.; Tang, B. A fluorescence nanosensor for glycoproteins with activity based on the molecularly imprinted spatial structure of the target and boronate affinity. Angew. Chem. Int. Ed. Engl. 2014, 53, 12489–12493. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.R.; Ni, Y.L.; Zhang, W.; Zhang, Z.Q.; Zhang, J. Detection of glycoprotein through fluorescent boronic acid-based molecularly imprinted polymer. Anal. Chim. Acta 2017, 960, 110–116. [Google Scholar] [CrossRef]
- Ye, J.; Chen, Y.; Liu, Z. A boronate affinity sandwich assay: An appealing alternative to immunoassays for the determination of glycoproteins. Angew. Chem. Int. Ed. Engl. 2014, 53, 10386–10389. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. Engl. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Such, G.K.; Johnston, A.P.R.; Liang, K.; Caruso, F. Synthesis and functionalization of nanoengineered materials using click chemistry. Prog. Polym. Sci. 2012, 37, 985–1003. [Google Scholar] [CrossRef]
- Hayase, G.; Kanamori, K.; Hasegawa, G.; Maeno, A.; Kaji, H.; Nakanishi, K. A superamphiphobic macroporous silicone monolith with marshmallow-like flexibility. Angew. Chem. Int. Ed. Engl. 2013, 52, 10788–10791. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Zhao, Y. Click chemistry as a versatile reaction for construction and modification of metal-organic frameworks. Coord. Chem. Rev. 2019, 380, 484–518. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.X.; Qing, P.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef]
- Bi, C.; Zhang, S.; Li, Y.; He, X.; Chen, L.; Zhang, Y. Boronic acid-functionalized iron oxide magnetic nanoparticles via distillation-precipitation polymerization and thiol-yne click chemistry for the enrichment of glycoproteins. New J. Chem. 2018, 42, 17331–17338. [Google Scholar] [CrossRef]
- Zhang, S.; He, X.; Chen, L.; Zhang, Y. Boronic acid functionalized magnetic nanoparticles via thiol–ene click chemistry for selective enrichment of glycoproteins. New J. Chem. 2014, 38, 4212. [Google Scholar] [CrossRef]
- Zhu, S.; Xia, M.; Chu, Y.; Khan, M.A.; Lei, W.; Wang, F.; Muhmood, T.; Wang, A. Adsorption and Desorption of Pb(II) on l-Lysine Modified Montmorillonite and the simulation of Interlayer Structure. Appl. Clay Sci. 2019, 169, 40–47. [Google Scholar] [CrossRef]
- Zhu, S.; Khan, M.A.; Kameda, T.; Xu, H.; Wang, F.; Xia, M.; Yoshioka, T. New insights into the capture performance and mechanism of hazardous metals Cr(3+) and Cd(2+) onto an effective layered double hydroxide based material. J. Hazard Mater. 2022, 426, 128062. [Google Scholar] [CrossRef] [PubMed]











| Adsorbate | Adsorbent | KD (mg/mL) | |
|---|---|---|---|
| High-Affinity Binding Sites | Low-Affinity Binding Sites | ||
| Adenosine | Fe3O4@MPTES@VPBA | 0.13 | 1.29 |
| Fe3O4@MPTES@ AAPBA | 0.05 | 1.95 | |
| Fe3O4@MPTES | — | 3.68 | |
| Fe3O4@MPS@MPBA | 0.05 | 1.33 | |
| Fe3O4@MPS | — | 3.45 | |
| o-Dihydroxybenzene | Fe3O4@MPTES@VPBA | 0.41 | 2.50 |
| Fe3O4@MPTES@ AAPBA | 0.33 | 2.64 | |
| Fe3O4@MPTES | — | 5.15 | |
| Fe3O4MPS@MPBA | 0.13 | 2.73 | |
| Fe3O4@MPS | — | 4.17 | |
| Adsorbate | Fe3O4@MPTES@VPBA | Fe3O4@MPTES@AAPBA | Fe3O4@MPS@MPBA | |||
|---|---|---|---|---|---|---|
| Qe (mg/g) | α | Qe (mg/g) | α | Qe (mg/g) | α | |
| Adenosine | 91.9 | - | 92.7 | - | 94.6 | - |
| Deoxyadenosine | 18.5 | 4.97 | 19.1 | 4.85 | 17.3 | 5.47 |
| o-Dihydroxybenzene | 80.6 | - | 79.3 | - | 83.1 | |
| m-Dihydroxybenzene | 34.6 | 2.33 | 36.4 | 2.18 | 28.5 | 2.92 |
| p-Dihydroxy-benzene | 31.1 | 2.59 | 32.6 | 2.43 | 26.2 | 3.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zhang, J.; Duan, A.; Wang, B.; Xie, S.; Yuan, L. Facile Preparation of Phenyboronic-Acid-Functionalized Fe3O4 Magnetic Nanoparticles for the Selective Adsorption of Ortho-Dihydroxy-Containing Compounds. Separations 2023, 10, 4. https://doi.org/10.3390/separations10010004
Zhou H, Zhang J, Duan A, Wang B, Xie S, Yuan L. Facile Preparation of Phenyboronic-Acid-Functionalized Fe3O4 Magnetic Nanoparticles for the Selective Adsorption of Ortho-Dihydroxy-Containing Compounds. Separations. 2023; 10(1):4. https://doi.org/10.3390/separations10010004
Chicago/Turabian StyleZhou, Hongmei, Junhui Zhang, Aihong Duan, Bangjin Wang, Shengming Xie, and Liming Yuan. 2023. "Facile Preparation of Phenyboronic-Acid-Functionalized Fe3O4 Magnetic Nanoparticles for the Selective Adsorption of Ortho-Dihydroxy-Containing Compounds" Separations 10, no. 1: 4. https://doi.org/10.3390/separations10010004
APA StyleZhou, H., Zhang, J., Duan, A., Wang, B., Xie, S., & Yuan, L. (2023). Facile Preparation of Phenyboronic-Acid-Functionalized Fe3O4 Magnetic Nanoparticles for the Selective Adsorption of Ortho-Dihydroxy-Containing Compounds. Separations, 10(1), 4. https://doi.org/10.3390/separations10010004