Fast Triacylglycerol Fingerprinting in Edible Oils by Subcritical Solvent Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Samples and Sample Preparation
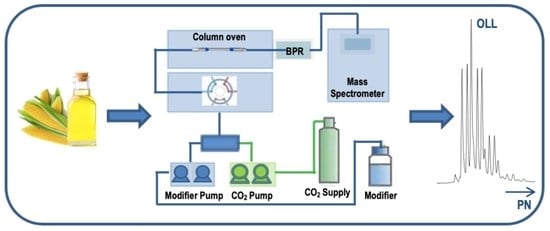
2.3. Instruments
2.4. Analytical Conditions
3. Results and Discussion
- -
- OOO/OLO/OLL/LLL/LLγLn/γLnLγLn/γLnγLnγLn, y = −0.0537x + 1.1497 (R2 = 0.9993).
- -
- SOO/SLO/SLL/SLLn, y = −0.0652x + 1.2051 (R2 = 0.9982);
- -
- OOP/OLP/OLnP/PLLn, y = −0.0555x + 1.0825 (R2 = 0.9993);
- -
- SOS/SOO/OOO/OLO/OOLn/OLLn/OLnLn, y = −0.0632x + 1.1978 (R2 = 0.992);
- -
- OOPo/OLPo/LLPo/γLnγLnP/γLnγLnPo, y = −0.0573x + 1.0769 (R2 = 0.9972);
- -
- SLS/SγLnS/SOγLn/SLγLn/SγLnγLn, y = −0.0600x + 1.1956 (R2 = 0.9963).
- -
- PPP/PLP/PLL/LLL, y = −0.0197x + 0.9389 (R2 = 0.9402);
- -
- SPP/SγLnP/SγLnγLn, y = −0.0257x + 1.0382 (R2 = 0.9753);
- -
- PPP/PγLnP/γLnγLnP/γLnγLnγLn, y = −0.0314x + 0.948 (R2 = 0.9983);
- -
- SPP/SLnP/SLnLn, y = −0.0357x + 1.0356 (R2 = 0.9861).
- -
- PPP/POM/PLM, y = −0.0652x + 0.9499 (R2 = 0.9998);
- -
- SPP/POP/PLP/OLM, y = −0.074x + 1.0352 (R2 = 0.9927);
- -
- SSP/SOP/OOP/OOPo/OLPo/LLPo/γLnγLnP/γLnγLnPo, y = −0.0632x + 1.1082 (R2 = 0.9957);
- -
- SOS/SOO/OOO/OLO/OLL/OLγLn/γLnLγLn/γLnγLnγLn, y = −0.0603x + 1.1904 (R2 = 0.9957);
- -
- GOS/GLS/GLO/GLL/GLγLn/GγLnγLn, y = −0.0595x + 1.2831 (R2 = 0.9922);
- -
- C22:1OS/C22:1OO/C22:1LO/C22:1LL/C22:1LγLn, y = −0.0636x + 1.3944 (R2 = 0.9943);
- -
- C24:1OS/C24:1LS/C24:1LO/C24:1LL/C24:1LγLn, y = −0.0664x + 1.5047 (R2 = 0.9924);
- -
- APP/AOP/AOO/BLO/LgLL/C22:1γLnC22:1, y = 0.0309x + 1.1166 (R2 = 0.9972);
- -
- SOP/SOO/ALO/BLL/C24:1LL, y = 0.0304x + 1.0172 (R2 = 0.9928);
- -
- POP/SLP/GLP/ALL/BLγLn/C24:1LγLn, y = 0.0328x + 0.9147 (R2 = 0.9959);
- -
- PLP/SLnP/SOLn/GOγLn/C22:1LγLn, y = 0.0344x + 0.8116 (R2 = 0.9902);
- -
- OLM/PLL/SLLn/C20:2LL, y = 0.0322x + 0.7216 (R2 = 0.9989).
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galanakis, C.M. Lipids and Edible Oils Properties, Processing and Applications, 1st ed.; Academic Press: London, UK, 2020. [Google Scholar]
- Wenk, M.R. Encyclopedia of Lipidomics, 1st ed.; Springer: Dordrecht, The Netherlands, 2022. [Google Scholar]
- Holčapek, M.; Liebisch, G.; Ekroos, K. Lipidomic Analysis. Anal. Chem. 2018, 90, 4249–4257. [Google Scholar] [CrossRef] [Green Version]
- Gyamfi, D.; Awuah, E.O.; Owusu, S. Lipid Metabolism: An Overview. In The Molecular Nutrition of Fats; Patel, V.B., Ed.; Academic Press: London, UK, 2019; pp. 17–32. [Google Scholar] [CrossRef]
- Brown, H.A.; Marnett, L.J. Introduction to lipid biochemistry, metabolism, and signaling. Chem Rev. 2011, 111, 5817–5820. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- James, W.; Elston, D.; Treat, J.; Rosenbach, M.; Micheletti, R. Nutritional Diseases. In Andrews’ Diseases of the Skin: Clinical Dermatology, 13th ed.; Elsevier: New York, NY, USA, 2019. [Google Scholar]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Gunstone, F.D. Vegetable Oils in Food Technology: Composition, Properties and Uses, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Lechhab, T.; Salmoun, F.; Lechhab, W.; El Majdoub, Y.O.; Russo, M.; Testa Camillo, M.R.; Trovato, E.; Dugo, P.; Mondello, L.; Cacciola, F. Determination of bioactive compounds in extra virgin olive oils from 19 Moroccan areas using liquid chromatography coupled to mass spectrometry: A study over two successive years. Eur. Food Res. Technol. 2021, 247, 2993–3012. [Google Scholar] [CrossRef]
- Indelicato, S.; Bongiorno, D.; Pitonzo, R.; Di Stefano, V.; Calabrese, V.; Indelicato, S.; Avellone, G. Triacylglycerols in edible oils: Determination, characterization, quantitation, chemometric approach and evaluation of adulterations. J. Chromatogr. A 2017, 1515, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tranchida, P.Q.; Donato, P.; Dugo, P.; Dugo, G.; Mondello, L. Comprehensive Chromatographic Methods for the Analysis of Lipids. Trends Anal. Chem. 2007, 26, 191–205. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Giannino, A.; Mondello, M.; Sciarrone, D.; Dugo, P.; Dugo, G.; Mondello, L. Elucidation of Fatty Acid Profiles in Vegetable Oils Exploiting Group-Type Patterning and Enhanced Sensitivity of Comprehensive Two-Dimensional Gas Chromatography. J. Sep. Sci. 2008, 31, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Tranchida, P.Q.; Costa, R.; Donato, P.; Sciarrone, D.; Ragonese, C.; Dugo, P.; Dugo, G.; Mondello, L. Acquisition of deeper knowledge on the human plasma fatty acid profile exploiting comprehensive 2-D GC. J Sep Sci. 2008, 31, 3347–3351. [Google Scholar] [CrossRef]
- Donato, P.; Micalizzi, G.; Oteri, M.; Rigano, F.; Sciarrone, D.; Dugo, P.; Mondello, L. Comprehensive lipid profiling in marine organisms by hyphenated and multidimensional chromatography techniques coupled to mass spectrometry detection. Anal. Bioanal. Chem. 2018, 410, 3297–3313. [Google Scholar] [CrossRef]
- Kallio, H.; Nylund, M.; Boström, P.; Yang, B. Triacylglycerol regioisomers in human milk resolved with an algorithmic novel electrospray ionization tandem mass spectrometry method. Food Chem. 2017, 233, 351–360. [Google Scholar] [CrossRef]
- Murphy, R.C. Challenges in Mass Spectrometry-based Lipidomics of Neutral Lipids. Trends Anal. Chem. 2018, 107, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, F.; Donato, P.; Sciarrone, D.; Dugo, P.; Mondello, L. Comprehensive Liquid Chromatography and Other Liquid-Based Comprehensive Techniques Coupled to Mass Spectrometry in Food Analysis. Anal. Chem. 2017, 89, 414–429. [Google Scholar] [CrossRef]
- Beccaria, M.; Sullini, G.; Cacciola, F.; Donato, P.; Dugo, P.; Mondello, L. High performance characterization of triacylglycerols in milk and milk-related samples by liquid chromatography and mass spectrometry. J. Chromatogr. A 2014, 1360, 172–187. [Google Scholar] [CrossRef]
- Lísa, M.; Denev, R.; Holčapek, M. Retention behaviour of isomeric triacylglycerols in silver-ion HPLC: Effects of mobile phase composition and temperature. J. Sep. Sci. 2013, 36, 2888–2900. [Google Scholar] [CrossRef]
- Lee, M.L.; Markides, K.E. Chromatography with supercritical fluids. Science 1987, 235, 1342–1347. [Google Scholar] [CrossRef]
- Donato, P.; Giuffrida, D.; Oteri, M.; Inferrera, V.; Dugo, P.; Mondello, L. Supercritical Fluid Chromatography × Ultra-High Pressure Liquid Chromatography for Red Chilli Pepper Fingerprinting by Photodiode Array, Quadrupole-Time-of-Flight and Ion Mobility Mass Spectrometry (SFC × RP-UHPLC-PDA-Q-ToF MS-IMS). Food Anal. Methods 2018, 11, 3331–3341. [Google Scholar] [CrossRef]
- Buchgraber, M.; Ulberth, F.; Emons, H.; Anklam, E. Triacylglycerol profiling by using chromatographic techniques. Eur. J. Lipid Sci. Technol. 2004, 106, 621–648. [Google Scholar] [CrossRef]
- Manninen, P.; Laakso, P.; Kallio, H. Method for characterization of triacylglycerols and fat-soluble vitamins in edible oils and fats by supercritical fluid chromatography. J. Am. Oil Chem. Soc. 1995, 72, 1001–1008. [Google Scholar] [CrossRef]
- Sandra, P.; Medvedovici, A.; Zhao, Y.; David, F. Characterization of triglycerides in vegetable oils by silver-ion packed-column supercritical fluid chromatography coupled to mass spectroscopy with atmospheric pressure chemical ionization and coordination ion spray. J. Chromatogr. A 2002, 974, 231–241. [Google Scholar] [CrossRef]
- Bernal, J.L.; Martín, M.T.; Toribio, L. Supercritical fluid chromatography in food analysis. J. Chromatogr. A 2013, 1313, 24–36. [Google Scholar] [CrossRef]
- Laboureur, L.; Ollero, M.; Touboul, D. Lipidomics by supercritical fluid chromatography. Int. J. Mol. Sci. 2015, 16, 13868–13884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesellier, E.; West, C. The many faces of packed column supercritical fluid chromatography—A critical review. J. Chromatogr. A 2015, 1382, 2–46. [Google Scholar] [CrossRef] [PubMed]
- Nováková, L.; Perrenoud, A.G.-G.; François, I.; West, C.; Lesellier, E.; Guillarme, D. Modern analytical supercritical fluid chromatography using columns packed with sub-2 particles: A tutorial. Anal. Chim. Acta 2014, 824, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, I.; Cacciola, F.; Utczas, M.; Inferrera, V.; Giuffrida, D.; Donato, P.; Dugo, P.; Mondello, L. Characterization of the pigment fraction in sweet bell peppers (Capsicum annuum L.) harvested at green and overripe yellow and red stages by offline multidimensional convergence chromatography/liquid chromatography-mass spectrometry. J. Sep. Sci. 2016, 39, 3281–3291. [Google Scholar] [CrossRef]
- Poole, C.F. Stationary phases for packed-column supercritical fluid chromatography. J. Chromatogr. A 2012, 1250, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Donato, P.; Inferrera, V.; Sciarrone, D.; Mondello, L. Supercritical fluid chromatography for lipid analysis in foodstuffs. J. Sep. Sci. 2017, 40, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Funada, Y.; Hirata, Y. Retention behavior of triglycerides in subcritical fluid chromatography with carbon dioxide mobile phase. J. Chromatogr. A 1997, 764, 301–307. [Google Scholar] [CrossRef]
- Lesellier, E.; Tchapla, A. Retention behavior of triglycerides in octadecyl packed subcritical fluid chromatography with CO2/modifier mobile phases. Anal. Chem. 1999, 71, 5372–5378. [Google Scholar] [CrossRef]
- Lísa, M.; Holčapek, M. Triacylglycerols profiling in plant oils important in food industry, dietetics and cosmetics using high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2008, 1198–1199, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Holčapek, M.; Lísa, M.; Jandera, P.; Kabátová, N. Quantitation of triacylglycerols in plant oils using HPLC with APCI-MS, evaporative light-scattering, and UV detection. J. Sep. Sci. 2005, 28, 1315–1333. [Google Scholar] [CrossRef] [PubMed]
- Mondello, L.; Beccaria, M.; Donato, P.; Cacciola, F.; Dugo, G.; Dugo, P. Comprehensive two-dimensional liquid chromatography with evaporative light scattering detection for the analysis of triacylglycerols in Borago Officinalis. J. Sep. Sci. 2011, 34, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Arena, P.; Sciarrone, D.; Dugo, P.; Donato, P.; Mondello, L. Pattern-Type Separation of Triacylglycerols by Silver Thiolate×Non-Aqueous Reversed Phase Comprehensive Liquid Chromatography. Separations 2021, 8, 88. [Google Scholar] [CrossRef]
- Bamba, T.; Matsubara, A.; Lee, J.W.; Fukusaki, E. Metabolic profiling of lipids by supercritical fluid chromatography/mass spectrometry. J. Chromatogr. A 2012, 1250, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Nishiumib, S.; Yoshida, M.; Fukusaki, E.; Bamba, T. Simultaneous profiling of polar lipids by supercritical fluid chromatography/tandem mass spectrometry with methylation. J. Chromatogr. A 2013, 1279, 98–107. [Google Scholar] [CrossRef] [PubMed]




| # | TAGs | PN | DB | [M + H]+exp. | Borage | Olive | Corn | Hazelnut | Palm | Peanut | Soybean |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | γLnγLnγLn | 36 | 9 | 873.7 | x | ||||||
| 2 | γLnγLnPo | 38 | 7 | 849.7 | x | ||||||
| 3 | LnLLn | 38 | 8 | 875.7 | x | ||||||
| 4 | γLnLγLn | 38 | 8 | 875.7 | x | ||||||
| 5 | LnLnP | 40 | 6 | 851.7 | x | ||||||
| 6 | γLnγLnP | 40 | 6 | 851.7 | x | ||||||
| 7 | LLLn | 40 | 7 | 877.7 | x | x | x | ||||
| 8 | LLγLn | 40 | 7 | 877.7 | x | ||||||
| 9 | LnOLn | 40 | 7 | 877.7 | x | ||||||
| 10 | γLnOγLn | 40 | 7 | 877.7 | x | ||||||
| 11 | LLPo | 42 | 5 | 853.7 | x | ||||||
| 12 | LnLP | 42 | 5 | 853.7 | x | x | x | ||||
| 13 | γLnLP | 42 | 5 | 853.7 | x | ||||||
| 14 | LLL | 42 | 6 | 879.7 | x | x | x | x | x | x | |
| 15 | OLLn | 42 | 6 | 879.7 | x | x | x | x | x | ||
| 16 | SLnLn | 42 | 6 | 879.7 | x | x | |||||
| 17 | OLγLn | 42 | 6 | 879.7 | x | ||||||
| 18 | SγLnγLn | 42 | 6 | 879.7 | x | ||||||
| 19 | GγLnγLn | 42 | 7 | 905.8 | x | ||||||
| 20 | PLM | 44 | 2 | 803.7 | x | ||||||
| 21 | PLnP | 44 | 3 | 829.7 | x | x | x | ||||
| 22 | PγLnP | 44 | 3 | 829.7 | x | ||||||
| 23 | OLM | 44 | 3 | 829.7 | x | ||||||
| 24 | LLP | 44 | 4 | 855.7 | x | x | x | x | x | x | x |
| 25 | OLPo | 44 | 4 | 855.7 | x | x | x | ||||
| 26 | LnOP | 44 | 4 | 855.7 | x | x | x | x | x | ||
| 27 | γLnOP | 44 | 4 | 855.7 | x | ||||||
| 28 | OLL | 44 | 5 | 881.7 | x | x | x | x | x | x | x |
| 29 | OOLn | 44 | 5 | 881.8 | x | x | x | x | |||
| 30 | SLLn | 44 | 5 | 881.8 | x | ||||||
| 31 | OOγLn | 44 | 5 | 881.8 | x | ||||||
| 32 | SLγLn | 44 | 5 | 881.8 | x | ||||||
| 33 | GLγLn | 44 | 6 | 907.8 | x | ||||||
| 34 | C20:2LL | 44 | 6 | 907.8 | x | ||||||
| 35 | PPM | 46 | 0 | 779.7 | x | ||||||
| 36 | POM | 46 | 1 | 805.7 | x | ||||||
| 37 | POPo | 46 | 2 | 831.7 | x | x | x | ||||
| 38 | PLP | 46 | 2 | 831.7 | x | x | x | x | x | x | x |
| 39 | OOM | 46 | 2 | 831.7 | x | ||||||
| 40 | OOPo | 46 | 3 | 857.8 | x | x | |||||
| 41 | OLP | 46 | 3 | 857.8 | x | x | x | x | x | x | x |
| 42 | SLnP | 46 | 3 | 857.8 | x | x | |||||
| 43 | SγLnP | 46 | 3 | 857.8 | x | ||||||
| 44 | OLO | 46 | 4 | 883.8 | x | x | x | x | x | x | x |
| 45 | SLL | 46 | 4 | 883.8 | x | x | x | x | |||
| 46 | SOLn | 46 | 4 | 883.8 | x | x | x | x | |||
| 47 | SOγLn | 46 | 4 | 883.8 | x | ||||||
| 48 | GγLnP | 46 | 4 | 883.8 | x | ||||||
| 49 | GLL | 46 | 5 | 909.8 | x | x | x | x | |||
| 50 | GOγLn | 46 | 5 | 909.8 | x | ||||||
| 51 | ALγLn | 46 | 5 | 909.8 | x | ||||||
| 52 | C22:1LγLn | 46 | 6 | 935.8 | x | ||||||
| 53 | PPP | 48 | 0 | 807.7 | x | x | |||||
| 54 | POP | 48 | 1 | 833.8 | x | x | x | x | x | x | x |
| 55 | OOP | 48 | 2 | 859.8 | x | x | x | x | x | x | x |
| 56 | SLP | 48 | 2 | 859.8 | x | x | x | x | x | x | |
| 57 | OOO | 48 | 3 | 885.8 | x | x | x | x | x | x | x |
| 58 | GLP | 48 | 3 | 885.8 | x | x | x | x | |||
| 59 | SLO | 48 | 3 | 885.8 | x | x | x | x | x | x | x |
| 60 | SγLnS | 48 | 3 | 885.8 | x | ||||||
| 61 | GγLnS | 48 | 4 | 911.8 | x | ||||||
| 62 | GLO | 48 | 4 | 911.8 | x | x | x | x | x | x | |
| 63 | ALL | 48 | 4 | 911.8 | x | x | x | x | |||
| 64 | C22:1γLnP | 48 | 4 | 911.8 | x | ||||||
| 65 | C22:1LL | 48 | 5 | 937.8 | x | x | |||||
| 66 | BLLn | 48 | 5 | 937.8 | x | ||||||
| 67 | BLγLn | 48 | 5 | 937.8 | x | ||||||
| 68 | C24:1LγLn | 48 | 6 | 963.8 | x | ||||||
| 69 | SPP | 50 | 0 | 835.8 | x | ||||||
| 70 | SOP | 50 | 1 | 861.8 | x | x | x | x | x | x | x |
| 71 | GOP | 50 | 2 | 887.8 | x | x | x | x | x | x | |
| 72 | ALP | 50 | 2 | 887.8 | x | x | x | x | x | x | |
| 73 | SLS | 50 | 2 | 887.8 | x | x | x | x | |||
| 74 | SOO | 50 | 2 | 887.8 | x | x | x | x | x | x | x |
| 75 | GOO | 50 | 3 | 913.8 | x | x | x | x | x | x | |
| 76 | ALO | 50 | 3 | 913.8 | x | x | x | x | x | x | |
| 77 | GLS | 50 | 3 | 913.8 | x | ||||||
| 78 | BγLnP | 50 | 3 | 913.8 | x | ||||||
| 79 | C22:1LO | 50 | 4 | 939.8 | x | x | |||||
| 80 | GLG | 50 | 4 | 939.8 | x | ||||||
| 81 | BLL | 50 | 4 | 939.8 | x | x | x | x | x | ||
| 82 | C24:1γLnP | 50 | 4 | 939.8 | x | ||||||
| 83 | C24:1OγLn | 50 | 5 | 965.9 | x | ||||||
| 84 | C24:1LL | 50 | 5 | 965.9 | x | ||||||
| 85 | LgLLn | 50 | 5 | 965.9 | x | ||||||
| 86 | C23:0LL | 51 | 4 | 953.9 | x | ||||||
| 87 | APP | 52 | 0 | 863.8 | x | ||||||
| 88 | SSP | 52 | 0 | 863.8 | x | ||||||
| 89 | AOP | 52 | 1 | 889.8 | x | x | x | x | x | x | |
| 90 | SOS | 52 | 1 | 889.8 | x | x | x | x | x | x | |
| 91 | GOS | 52 | 2 | 915.8 | x | ||||||
| 92 | AOO | 52 | 2 | 915.8 | x | x | x | x | x | x | x |
| 93 | BLP | 52 | 2 | 915.8 | x | x | x | x | |||
| 94 | ALS | 52 | 2 | 915.8 | x | x | x | ||||
| 95 | C22:1OP | 52 | 2 | 915.8 | x | ||||||
| 96 | C22:1OO | 52 | 3 | 941.9 | x | x | |||||
| 97 | C24:1LP | 52 | 3 | 941.9 | x | ||||||
| 98 | BLO | 52 | 3 | 941.9 | x | x | x | x | x | ||
| 99 | C24:1LO | 52 | 4 | 967.9 | x | ||||||
| 100 | LgLL | 52 | 4 | 967.9 | x | x | x | x | x | ||
| 101 | C24:1γLnS | 52 | 4 | 967.9 | x | ||||||
| 102 | C22:1γLnC22:1 | 52 | 5 | 993.9 | x | ||||||
| 103 | BOP | 54 | 1 | 917.9 | x | x | x | ||||
| 104 | AOS | 54 | 1 | 917.9 | x | x | x | ||||
| 105 | C22:1OS | 54 | 2 | 943.9 | x | ||||||
| 106 | C24:1OP | 54 | 2 | 943.9 | x | ||||||
| 107 | BOO | 54 | 2 | 943.9 | x | x | x | x | x | x | |
| 108 | LgLP | 54 | 2 | 943.9 | x | x | x | x | |||
| 109 | BLS | 54 | 2 | 943.9 | x | x | |||||
| 110 | C24:1OO | 54 | 3 | 969.9 | x | ||||||
| 111 | C24:1LS | 54 | 3 | 969.9 | x | ||||||
| 112 | C22:1OG | 54 | 3 | 969.9 | x | ||||||
| 113 | LgLO | 54 | 3 | 969.9 | x | x | x | x | x | x | |
| 114 | C23:0OO | 55 | 2 | 957.9 | x | ||||||
| 115 | LgOP | 56 | 1 | 945.9 | x | ||||||
| 116 | BOS | 56 | 1 | 945.9 | x | ||||||
| 117 | LgOO | 56 | 2 | 971.9 | x | x | x | x | x | ||
| 118 | LgLS | 56 | 2 | 971.9 | x | ||||||
| 119 | C24:1OS | 56 | 2 | 971.9 | x | ||||||
| 120 | C22:1OC22:1 | 56 | 3 | 997.9 | x | ||||||
| 121 | C24:1OG | 56 | 3 | 997.9 | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salerno, T.M.G.; Oteri, M.; Arena, P.; Trovato, E.; Sciarrone, D.; Donato, P.; Mondello, L. Fast Triacylglycerol Fingerprinting in Edible Oils by Subcritical Solvent Chromatography. Separations 2023, 10, 56. https://doi.org/10.3390/separations10010056
Salerno TMG, Oteri M, Arena P, Trovato E, Sciarrone D, Donato P, Mondello L. Fast Triacylglycerol Fingerprinting in Edible Oils by Subcritical Solvent Chromatography. Separations. 2023; 10(1):56. https://doi.org/10.3390/separations10010056
Chicago/Turabian StyleSalerno, Tania Maria Grazia, Marianna Oteri, Paola Arena, Emanuela Trovato, Danilo Sciarrone, Paola Donato, and Luigi Mondello. 2023. "Fast Triacylglycerol Fingerprinting in Edible Oils by Subcritical Solvent Chromatography" Separations 10, no. 1: 56. https://doi.org/10.3390/separations10010056
APA StyleSalerno, T. M. G., Oteri, M., Arena, P., Trovato, E., Sciarrone, D., Donato, P., & Mondello, L. (2023). Fast Triacylglycerol Fingerprinting in Edible Oils by Subcritical Solvent Chromatography. Separations, 10(1), 56. https://doi.org/10.3390/separations10010056