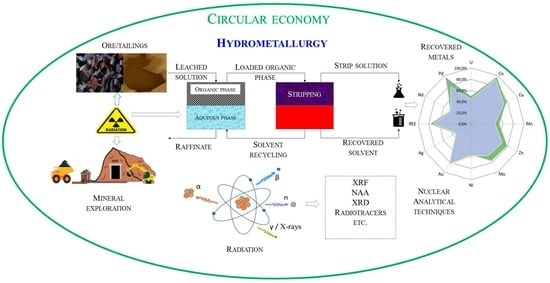
The State of Critical and Strategic Metals Recovery and the Role of Nuclear Techniques in the Separation Technologies Development: Review
Abstract
:1. Introduction
1.1. Critical and Strategic Metals
1.2. Critical Metals
1.3. Strategic Metals
1.4. Current Progress in Nuclear Technologies
1.5. Recovery of the Metals
2. Role of Nuclear Technologies and Nuclear Analytical Methods in the Advancement of Hydrometallurgical Processes
- −
- the method of isotope tracers for studying the physicochemical foundations of processes; laboratory industrial technology studies, a method for researching the effectiveness of existing industrial technologies and apparatus; and the control and regulation of industrial processes;
- −
- radiometric analysis, such as the neutron activation method, X-Ray Fluorescence spectrometry, and others, due to the possibility of rapid analysis without destroying the tested material and the possibility of operating with very low concentrations;
- −
- the use of nuclear equipment to control and regulate technological processes.
2.1. Development of Nuclear Techniques for the Study of Metals and Recovery Using Hydrometallurgical Processes
2.1.1. Neutron Activation Analysis (NAA) of the metals
2.1.2. XRF Spectrometry
2.1.3. XRD
3. The Radiotracer Methods
3.1. Application of Radiotracer Techniques in the Optimization of the Chemical Processes in Hydrometallurgy Using RTD
3.2. Application of the Radiotracer Techniques in Leaching
3.3. Application of Radiotracers in Solvent Extraction of Metals
- −
- E is the extraction coefficient;
- −
- I0 is the number of counts in the organic phase of volume V;
- −
- Iw is the number of counts in the water phase of the same volume V;
- −
- It is the background of the measuring device.

3.4. Application of Radiotracers in the Adsorption Processes of Metal Recovery
3.5. Radiotracer Studies Using Resins
- −
- A0 is the solution count rate prior to ion exchanger equilibration;
- −
- A is the count rate of solvent following ion exchanger equilibration;
- −
- L is the solution’s volume (mL);
- −
- m is the mass of ion exchange resin.
4. Product Analyses
- −
- is the activity of the excited radionuclide excited due to irradiation of the sample with neutrons; activity is expressed in [Bq], which is the number of decays per second;
- −
- is the atomic number of a given isotope in the target material;
- −
- is the neutron flux density (cm−2s−1);
- −
- is the activation cross-section, which is the probability of a nuclear reaction occurring expressed in ;
- −
- t is the irradiation time [s];
- −
- is the radioactive decay constant: ;
- −
- is the half-life of the radionuclide [s];
- −
- is the saturation factor, representing the fraction of the maximum obtainable activity after irradiation.
- −
- x is the concentration of the determined element (ppm);
- −
- P is the number of counts collected in a given peak;
- −
- D is the correction for nuclide decay before starting the measurements;
- −
- C is the factor responsible for the decay of the radionuclide during the measurements;
- −
- is the sample weight [g];
- −
- is the mass of the standard [µg];
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tausova, M.; Culkova, K.; Domaracká, L.; Drebenstedt, C.; Taušová, M.; Čulková, K.; Muchová, M.S.; Koščo, J.; Behúnová, A.; Drevková, M.; et al. The importance of mining for socio-economic growth of the country. Acta Montan. Slovaca 2017, 22, 359–367. [Google Scholar]
- Ascher, W. Why Governments Waste Natural Resources: Policy Failures in Developing Countries; JHU Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Wojciech, K. MINLEX -Poland Country Report; MinPol and partner: Warsaw, Poland, 2019; pp. 1–95. Available online: https://rmis.jrc.ec.europa.eu/uploads/legislation/MINLEX_CountryReport_PL.pdf (accessed on 17 October 2022).
- Maina, D.M.; Ndirangu, D.M.; Mangala, M.M.; Boman, J.; Shepherd, K.; Gatari, M.J. Environmental implications of high metal content in soils of a titanium mining zone in Kenya. Environ. Sci. Pollut. Res. 2016, 23, 21431–21440. [Google Scholar] [CrossRef] [PubMed]
- Ceniceros-Gómez, A.E.; Macías-Macías, K.Y.; de la Cruz-Moreno, J.E.; Gutiérrez-Ruiz, M.E.; Martínez-Jardines, L.G. Characterization of mining tailings in México for the possible recovery of strategic elements. J. South Am. Earth Sci. 2018, 88, 72–79. [Google Scholar] [CrossRef]
- Kirsten, H.; Daniele, L.P.; Thao, F.; Tim, L.; John, D. Minerals for Climate Action: The Mineral Intensity of the Clean Energy Transition; The World Bank: Washington, WA, USA, 2020; pp. 1–112. Available online: https://pubdocs.worldbank.org/en/961711588875536384/Minerals-for-Climate-Action-The-Mineral-Intensity-of-the-Clean-Energy-Transition.pdf (accessed on 27 January 2023).
- Kim, J.; Guillaume, B.; Chung, J.; Hwang, Y. Critical and precious materials consumption and requirement in wind energy system in the EU 27. Appl. Energy 2015, 139, 327–334. [Google Scholar] [CrossRef]
- Grandell, L.; Lehtilä, A.; Kivinen, M.; Koljonen, T.; Kihlman, S.; Lauri, L.S. Role of critical metals in the future markets of clean energy technologies. Renew. Energy 2016, 95, 53–62. [Google Scholar] [CrossRef]
- Moss, R.L.; Tzimas, E.; Kara, H.; Willis, P.; Kooroshy, J. Critical Metals in Strategic Energy Technologies: Assessing rare metals as supply-chain bottlenecks in low-carbon energy technologies. In JRC-scientific and strategic reports, European Commission Joint Research Centre Institute for Energy and Transport; Institute for Energy and Transport IET: Hague, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, Y.; Liu, B.; Chang, C.C. Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag. 2017, 65, 113–127. [Google Scholar] [CrossRef]
- Habib, K.; Hansdóttir, S.T.; Habib, H. Critical metals for electromobility: Global demand scenarios for passenger vehicles, 2015–2050. Resour. Conserv. Recycl. 2020, 154, 104603. [Google Scholar] [CrossRef]
- Blengini, G.A.; Mathieux, F.; Mancini, L.; Nyberg, M.; Cavaco Viegas, H.; Salminen, J.; Garbarino, E.; Orveillion, G.; Saveyn, H. Recovery of critical and other raw materials from mining waste and landfills. In JRC Science for Policy Report; European Commission: Maastricht, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Ayres, R.U.; Peiró, L.T. Material efficiency: Rare and critical metals. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20110563. [Google Scholar] [CrossRef]
- Žibret, G.; Lemiere, B.; Mendez, A.M.; Cormio, C.; Sinnett, D.; Cleall, P.; Szabo, K.; Carvalho, T. National mineral waste databases as an information source for assessing material recovery potential from mine waste, tailings and metallurgical waste. Minerals 2020, 10, 446. [Google Scholar] [CrossRef]
- Purwadi, I.; van der Werff, H.M.A.; Lievens, C. Targeting rare earth element bearing mine tailings on Bangka Island, Indonesia, with Sentinel-2 MSI. Int. J. Appl. Earth Obs. Geoinf. 2020, 88, 102055. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Geochemical fractions of rare earth elements in soil around a mine tailing in Baotou, China. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.A. Rare earth metals: A strategic concern. Miner. Econ. 2014, 27, 21–31. [Google Scholar] [CrossRef]
- Zhuang, W.Q.; Fitts, J.P.; Ajo-Franklin, C.M.; Maes, S.; Alvarez-Cohen, L.; Hennebel, T. Recovery of critical metals using biometallurgy. Curr. Opin. Biotechnol. 2015, 33, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, G.J.P.; Chagnes, A.; Cote, G. Recent Advances in the chemistry of hydrometallurgical methods. Sep. Purif. Rev. 2022, 1. [Google Scholar] [CrossRef]
- Seetharaman, S.; McLean, A.; Guthrie, R.; Sridhar, S. Treatise on Process Metallurgy, Volume 3: Industrial processes; Newnes: Tokyo, Japan, 2013; Volume 3. [Google Scholar]
- Galos, K.; Kot-Niewiadomska, A.; Kamyk, J. The Role of Poland in the European Union Supply Chain of Raw Materials, Including Critical Raw Materials. Mater. Proc. 2021, 5, 14. [Google Scholar] [CrossRef]
- Fleming, P.; Orrego, P.; Pinilla, F. Recovery of Rare Earth Elements Present in Mining Tails, by Leaching with Nitric and Hydrochloric Solutions. World J. Nucl. Sci. Technol. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Smith, M.P.; Kynicky, J. From “strategic” tungsten to “green” neodymium: A century of critical metals at a glance. Ore Geol. Rev. 2015, 64, 455–458. [Google Scholar] [CrossRef]
- House of Commons. House of Commons Science and Technology Committee: HC 726; Strategically Important Metals; Fifth Report of Session 2010-12; 2011. Available online: www.parliament.uk/science (accessed on 20 October 2022).
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research—global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Field, F.R.; Wallington, T.J.; Everson, M.; Kirchain, R.E. Strategic Materials in the Automobile: A Comprehensive Assessment of Strategic and Minor Metals Use in Passenger Cars and Light Trucks. Environ. Sci. Technol. 2017, 51, 14436–14444. [Google Scholar] [CrossRef]
- Zuo, L.; Wang, C.; Corder, G.D. Strategic evaluation of recycling high-tech metals from urban mines in China: An emerging industrial perspective. J. Clean. Prod. 2019, 208, 697–708. [Google Scholar] [CrossRef]
- Mishra, B.; Anderson, C.D.; Taylor, P.R.; Anderson, C.G.; Apelian, D.; Blanpain, B. CR3 update: Recycling of strategic metals. Jom 2012, 64, 441–443. [Google Scholar] [CrossRef]
- Calvo, G.; Valero, A.; Valero, A. How can strategic metals drive the economy? Tungsten and tin production in Spain during periods of war. Extr. Ind. Soc. 2019, 6, 8–14. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Rajesh Kumar, J.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Giani, M.I.; Recanati, F.; Dotelli, G.; Puricelli, S.; Cristiani, C. Environmental impacts of a hydrometallurgical process for electronic waste treatment: A life cycle assessment case study. J. Clean. Prod. 2017, 140, 1204–1216. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. The Current Status and Future Trends on Radioisotope Applications in Industry. Expert Rev. Med. Devices 2012, 7, 331–343. [Google Scholar]
- Pant, H.J. Applications of the radiotracers in the industry: A review. Appl. Radiat. Isot. 2022, 182, 110076. [Google Scholar] [CrossRef]
- Teviso Sensor Technologies Ltd. 2023. Available online: https://www.teviso.com/ (accessed on 27 January 2023).
- Company | TD-Electronics. 2023. Available online: https://td-electronics.pl/en/company/ (accessed on 27 January 2023).
- CSIRO. Commonwealth Scientific and Industrial Research Organisation, Australian Government; CSIRO: Canberra, Australia, 2023. [Google Scholar]
- Li, M.; Deng, R.; Cao, J.; Qiu, J.; Lei, G.; Li, X.; Zhang, B. Research on remaining oil evaluation method based on cased hole logging technology. Energy Rep. 2021, 7, 1168–1174. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, F.; Tian, L.; Liang, Q.; Zhang, X.; Fang, Q.; Lu, B.; Li, X. A method of monitoring gas saturation in carbon dioxide injection heavy oil reservoirs by pulsed neutron logging technology. Pet. Explor. Dev. 2021, 48, 1420–1429. [Google Scholar] [CrossRef]
- Pant, H.J.; Kundu, A.; Nigam, K.D.P. Radiotracer applications in chemical process industry. Rev. Chem. Eng. 2001, 17, 165–252. [Google Scholar] [CrossRef]
- Vanhoof, C.; Bacon, J.R.; Fittschen, U.E.A.; Vincze, L. Atomic spectrometry update—A review of advances in X-ray fluorescence spectrometry and its special applications. J. Anal. At. Spectrom. 2021, 36, 1797–1812. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Zhou, W.; Mo, G.; Chen, H.; Xu, T. Review on the elemental analysis of polymetallic deposits by total-reflection X-ray fluorescence spectrometry. Appl. Spectrosc. Rev. 2022, 1–14. [Google Scholar] [CrossRef]
- Khan, H.; Yerramilli, A.S.; D’Oliveira, A.; Alford, T.L.; Boffito, D.C.; Patience, G.S. Experimental methods in chemical engineering: X-ray diffraction spectroscopy—XRD. Can. J. Chem. Eng. 2020, 98, 1255–1266. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-Ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Feng, X.; Onel, O.; Council-Troche, M.; Noble, A.; Yoon, R.H.; Morris, J.R. A study of rare earth ion-adsorption clays: The speciation of rare earth elements on kaolinite at basic pH. Appl. Clay Sci. 2021, 201, 105920. [Google Scholar] [CrossRef]
- Habashi, F. A short history of hydrometallurgy. Hydrometallurgy 2005, 79, 15–22. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Dreisinger, D.B.; Espinosa, D.C.R. A Review of Nickel, Copper, and Cobalt Recovery by Chelating Ion Exchange Resins from Mining Processes and Mining Tailings. Min. Metall. Explor. 2019, 36, 199–213. [Google Scholar] [CrossRef]
- Florek, J.; Giret, S.; Juère, E.; Larivière, D.; Kleitz, F. Functionalization of mesoporous materials for lanthanide and actinide extraction. Dalton Trans. 2016, 45, 14832. [Google Scholar] [CrossRef]
- Um, N.; Um, N. Hydrometallurgical Recovery Process of Rare Earth Elements from Waste: Main Application of Acid Leaching with Devised τ-T Diagram. Rare Earth Elem. 2017, 44–58. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, C.Y. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts: Part I: Metallurgical processes. Hydrometallurgy 2009, 98, 1–9. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Y.; Lin, J.; Yue, Z.; Li, W.; Jin, J.; Sun, Q.; Ai, Y.; Feng, M.; Huang, X. Highly Selective Recovery of Lanthanides by Using a Layered Vanadate with Acid and Radiation Resistance. Angew. Chem. 2020, 132, 1894–1899. [Google Scholar] [CrossRef]
- Rogowski, M.; Smolinski, T.; Pyszynska, M.; Brykala, M.; Chmielewski, A.G. Studies on hydrometallurgical processes using nuclear techniques to be applied in copper industry. II. Application of radiotracers in copper leaching from flotation tailings. Nukleonika 2018, 63, 131–137. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Narbutt, J.; Chmielewski, A.G. Stan Obecny Oraz Perspektywy Rozwoju Radiochemii i Chemii Jądrowej w Polsce. 2001. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Jerzy+Narbutt%2C+Andrzej+G.+Chmielewski%2C+STAN+OBECNYORAZ+PERSPEKTYWY+ROZWOJU+RADIOCHEMII+I+CHEMII+JĄDROWEJ+W++POLSCE%2C+RAPORTY+IChTJ.+SERIA+B+nr+1%2F2001&btnG= (accessed on 13 April 2022).
- Dybczyński, R.S. The role of NAA in securing the accuracy of analytical results in the inorganic trace analysis. J. Radioanal. Nucl. Chem. 2019, 322, 1505–1515. [Google Scholar] [CrossRef]
- Yao, M.; Wang, D.; Zhao, M. Element Analysis Based on Energy-Dispersive X-Ray Fluorescence. Adv. Mater. Sci. Eng. 2015, 2015, 290593. [Google Scholar] [CrossRef]
- Regadío, M.; Riañ, S.; Binnemans, K.; Hoogerstraete, T.V. Direct Analysis of Metal Ions in Solutions with High Salt Concentrations by Total Reflection X-ray Fluorescence. Anal. Chem. 2017, 89, 4595–4603. [Google Scholar] [CrossRef]
- Omran, E.; Abd, A.A.; Razek, E. Mapping and screening risk assessment of heavy metals concentrations in soils of the Bahr El-Baker Region, Egypt. Undefined 2012, 3, 182–195. [Google Scholar] [CrossRef]
- Przewlocki, K.; Petryka, L.; Stḙgowski, Z. Radiotracer Investigation of the Copper Ore Concentration Process. In Isotopenpraxis Isotopes in Environmental and Health Studies; Taylor & Francis Group: Abingdon, UK, 1990; pp. 439–444. [Google Scholar] [CrossRef]
- Nkuna, R.; Ijoma, G.N.; Matambo, T.S.; Chimwani, N. Accessing Metals from Low-Grade Ores and the Environmental Impact Considerations: A Review of the Perspectives of Conventional versus Bioleaching Strategies. Minerals 2022, 12, 506. [Google Scholar] [CrossRef]
- Nad, A.; Jooshaki, M.; Tuominen, E.; Michaux, S.; Kirpala, A.; Newcomb, J. Digitalization Solutions in the Mineral Processing Industry: The Case of GTK Mintec, Finland. Minerals 2022, 12, 210. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. TECHNOLOGY TRANSFER OF NUCLEAR TECHNIQUES AND NUCLEONIC CONTROL SYSTEMS IN THE MINERAL INDUSTRY. 1990. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/te_0578.pdf (accessed on 18 October 2022).
- Brisset, P.; Miskovic, S. Development of Radiometric Methods for Exploration and Process Optimization in Mining and Mineral Industries. IAEA Vienna Sept. 2014, pp. 1–5. Available online: http://www-naweb.iaea.org/napc/iachem/working_materials/CM7%20Meeting%20on%20preparation%20of%20a%20CRP%20in%20Mining%20industry%20-%20Report%20-%20Final.pdf (accessed on 18 October 2022).
- Kraś, J.; Nobis, C.; Myczkowski, S. Leakage Control Methods for Metal Underground Tanks and Tanks Placed on Hardened Soil with the Use of Radioactive Tracers. Nukleonika 2008, 2, 137–140. Available online: https://www.infona.pl//resource/bwmeta1.element.baztech-3162594f-579f-439a-934c-eb34be8a80a0 (accessed on 24 October 2022).
- Petryka, L.; Przewlocki, K. Optimization of Copper Ore Concentration Processing by Means of Radioactive Tracers. Isot. Isot. Environ. Health Stud. 1989, 25, 139–143. [Google Scholar] [CrossRef]
- Thereska, J.; Çuçi, T.; Plasari, E.; Lleshi, Z. Radiotracer determination of gold recovery in copper melting process. J. Radioanal. Nucl. Chem. Lett. 1989, 136, 197–202. [Google Scholar] [CrossRef]
- Michalik, J.S.; Palige, J.; Bazaniak, Z. Radiotracer Investigations of the Shaft Processes in Polish Zine and Lead Metallurgy Part. I. Investigation of Charge Materials Movement. Isot. Isot. Environ. Health Stud. 1990, 26, 210–215. [Google Scholar] [CrossRef]
- Bazaniak, Z.; Klimkiewicz, T. Studies on Reduction Process of Cu2O in an Electric Furnace Using Radiotracers; Taylor & Francis: Abingdon, UK, 1983; Volume 19, pp. 373–376. [Google Scholar] [CrossRef]
- Rosenberg, R.J.; Guizerix, J. Nuclear techniques for peaceful development Nuclear techniques in mineral exploration, extraction, and processing Overview of typical applications and the IAEA’s activities in the field. IAEA Bull. 1987, 29, 28–32. [Google Scholar]
- Glascock, M.D. An Overview of Neutron Activation Analysis; University of Missouri Research Reactor (MURR): Columbia, MO, USA, 2006. [Google Scholar]
- El-Taher, A.; Kratz, K.L.; Nossair, A.; Azzam, A.H. Determination of gold in two Egyptian gold ores using instrumental neutron activation analysis. Radiat. Phys. Chem. 2003, 68, 751–755. [Google Scholar] [CrossRef]
- El-Taher, A. Elemental analysis of two Egyptian phosphate rock mines by instrumental neutron activation analysis and atomic absorption spectrometry. Appl. Radiat. Isot. 2010, 68, 511–515. [Google Scholar] [CrossRef]
- Nandy, M.; Nandy, M. Neutron Activation Analysis: Application in Geology and Medicine. In Advanced Technologies and Applications of Neutron Activation Analysis; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Zovko, E.; Islamović, S. Application of neutron activation in hydrometallurgical process of lead chloride extraction from boulangerit. Hem. Ind. 2010, 64, 53–55. [Google Scholar] [CrossRef]
- Smolik, M.; Polkowska-Motrenko, H.; Hubicki, Z.; Jakóbik-Kolon, A.; Danko, B. Determination of hafnium at the 10-4% level (relative to zirconium content) using neutron activation analysis, inductively coupled plasma mass spectrometry and inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta 2014, 806, 97–100. [Google Scholar] [CrossRef]
- Osawa, T.; Hatsukawa, Y.; Appel, P.W.U.; Matsue, H. Mercury and gold concentrations of highly polluted environmental samples determined using prompt gamma-ray analysis and instrument neutron activation analysis. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2011, 269, 717–720. [Google Scholar] [CrossRef]
- Rotich, N.K. Evaluation of the Essential Trace Metals in Soils and African Spider Plants: Case Study of Molo Ward-Nakuru County [University of Nairobi]. 2019. Available online: http://erepository.uonbi.ac.ke/handle/11295/108854 (accessed on 31 October 2022).
- Brouwer, P. Theory of X-ray Fluorescence. Getting Acquainted with the Principles, 3rd ed.; PANalytical, B.V., Ed.; PANanalytical: Malvern, UK, 2013. [Google Scholar]
- Goldstein, J.I.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Lyman, C.E.; Lifshin, E.; Sawyer, L.; Michael, J.R. Scanning Electron Microscopy and X-ray Microanalysis and analytical ELECTRON Microscopy; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Kuhn, K.; Meima, J.A.; Rammlmair, D.; Ohlendorf, C. Chemical mapping of mine waste drill cores with laser-induced breakdown spectroscopy (LIBS) and energy dispersive X-ray fluorescence (EDXRF) for mineral resource exploration. J. Geochem. Explor. 2015, 161, 72–84. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, O.; Queralt, I.; Carvalho, M.L.; Garcia, G. Elemental analysis of mining wastes by energy dispersive X-ray fluorescence (EDXRF). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 262, 81–86. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, O.; Queralt, I. Fast elemental screening of soil and sediment profiles using small-spot energy-dispersive X-ray fluorescence: Application to mining sediments geochemistry. Appl. Spectrosc. 2010, 64, 1045–1053. [Google Scholar] [CrossRef]
- Remya Devi, P.S.; Trupti, A.C.; Nicy, A.; Dalvi, A.A.; Swain, K.K.; Wagh, D.N.; Verma, R. Evaluation of uncertainty in the energy dispersive X-ray fluorescence determination of platinum in alumina. Anal. Methods 2015, 7, 5345–5351. [Google Scholar] [CrossRef]
- Craddock, P.R.; Herron, M.M.; Herron, S.L. COMPARISON OF QUANTITATIVE MINERAL ANALYSIS BY X-RAY DIFFRACTION AND FOURIER TRANSFORM INFRARED SPECTROSCOPY. J. Sediment. Res. 2017, 87, 630–652. [Google Scholar] [CrossRef]
- Majuste, D.; Ciminelli, V.S.T.; Eng, P.J.; Osseo-Asare, K. Applications of in situ synchrotron XRD in hydrometallurgy: Literature review and investigation of chalcopyrite dissolution. Hydrometallurgy 2013, 131–132, 54–66. [Google Scholar] [CrossRef]
- Stevie, F.A.; Donley, C.L. Introduction to x-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A Vac. Surf. Film. 2020, 38, 063204. [Google Scholar] [CrossRef]
- Yano, J.; Yachandra, V.K. X-ray absorption spectroscopy. Photosynth. Res. 2009, 102, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.A.; Adekola, F.A. Hydrometallurgical processing of a Nigerian sphalerite in hydrochloric acid: Characterization and dissolution kinetics. Hydrometallurgy 2010, 101, 69–75. [Google Scholar] [CrossRef]
- Majuste, D.; Ciminelli, V.S.T.; Osseo-Asare, K.; Dantas, M.S.S.; Magalhães-Paniago, R. Electrochemical dissolution of chalcopyrite: Detection of bornite by synchrotron small angle X-ray diffraction and its correlation with the hindered dissolution process. Hydrometallurgy 2012, 111–112, 114–123. [Google Scholar] [CrossRef]
- Xia, J.L.; Song, J.J.; Liu, H.C.; Nie, Z.Y.; Shen, L.; Yuan, P.; Ma, C.Y.; Zheng, L.; Zhao, Y.D. Study on catalytic mechanism of silver ions in bioleaching of chalcopyrite by SR-XRD and XANES. Hydrometallurgy 2018, 180, 26–35. [Google Scholar] [CrossRef]
- Watari, T.; Nansai, K.; Nakajima, K. Review of critical metal dynamics to 2050 for 48 elements. Resour. Conserv. Recycl. 2020, 155, 104669. [Google Scholar] [CrossRef]
- Kim, W.J.; Seo, S.; Lee, S.I.; Kim, D.W.; Kim, M.J. A study on pyro-hydrometallurgical process for selective recovery of Pb, Sn and Sb from lead dross. J. Hazard. Mater. 2021, 417, 126071. [Google Scholar] [CrossRef]
- Rabatho, J.P.; Tongamp, W.; Takasaki, Y.; Haga, K.; Shibayama, A. Recovery of Nd and Dy from rare earth magnetic waste sludge by hydrometallurgical process. J. Mater. Cycles Waste Manag. 2012, 15, 171–178. [Google Scholar] [CrossRef]
- Han, H.; Sun, W.; Hu, Y.; Cao, X.; Tang, H.; Liu, R.; Yue, T. Magnetite precipitation for iron removal from nickel-rich solutions in hydrometallurgy process. Hydrometallurgy 2016, 165, 318–322. [Google Scholar] [CrossRef]
- Pathak, N.; Sen, R. Analytical Application of Radioactive Analysis. Int. J. Anal. Appl. Chem. 2017, 3, 32–35. Available online: https://chemical.journalspub.info/index.php?journal=JAAC&page=article&op=view&path%5B%5D=410 (accessed on 18 October 2022).
- Gitau, J.; Gatari, M.J.; Pant, H.J. Investigation of flow dynamics of porous clinkers in a ball mill using technitium-99m as a radiotracer. Appl. Radiat. Isot. 2019, 154, 108902. [Google Scholar] [CrossRef]
- Othman, N.; Kamarudin, S.K. Radiotracer technology in mixing processes for industrial applications. Sci. World J. 2014, 2014, 768604. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, M.; Chandra, A.; Bhunia, H.; Bajpai, P.K.; Pant, H.J. Residence time distribution studies using radiotracers in chemical industry—A review. Chem. Eng. Commun. 2018, 205, 739–758. [Google Scholar] [CrossRef]
- el Korchi, K.; Alami, R.; Saadaoui, A.; Mimount, S.; Chaouch, A. Residence time distribution studies using radiotracers in a lab-scale distillation column: Experiments and modeling. Appl. Radiat. Isot. 2019, 154, 108889. [Google Scholar] [CrossRef]
- Din, G.U.; Khan, I.H.; Chughtai, I.R.; Inayat, M.H.; Jin, J.H. Radiotracer investigations to study the hydrodynamic characteristics of continuous phase in a pulsed sieve plate extraction column. EPJ Web Conf. 2013, 50, 01004. [Google Scholar] [CrossRef]
- Pant, H.J.; Sharma, V.K.; Naik, S.V.; Singh, G.; Kalgutkar, D.B.; Patil, S.P.; Jayachandran, N.; Unni, V.K.P. Investigation of leaching of an antifouling agent from marine paint formulations using radiotracer technique. J. Radioanal. Nucl. Chem. 2012, 294, 65–69. [Google Scholar] [CrossRef]
- Goswami, S.; Biswal, J.; Samantray, J.; Gupta, D.F.; Pant, H.J. Measurement of mixing time and holdup of solids in gas–solid fluidized bed using radiotracer technique. J. Radioanal. Nucl. Chem. 2014, 302, 845–850. [Google Scholar] [CrossRef]
- Kasban, H.; Arafa, H.; Elaraby, S.M.S. Principle component analysis for radiotracer signal separation. Appl. Radiat. Isot. 2016, 112, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.J.; Sharma, V.K.; Singh, G.; Raman, V.K.; Bornare, J.; Sonde, R.R. Radiotracer investigation in a rotary fluidized bioreactor. J. Radioanal. Nucl. Chem. 2012, 294, 59–63. [Google Scholar] [CrossRef]
- Pant, H.J.; Sharma, V.K.; Shenoy, K.T.; Sreenivas, T. Measurements of liquid phase residence time distributions in a pilot-scale continuous leaching reactor using radiotracer technique. Appl. Radiat. Isot. 2015, 97, 40–46. [Google Scholar] [CrossRef]
- Domínguez, J.; Abreu, A.M.; McCalla, R.; Borroto, J.; Ortueta, M.; Pérez, E. Mixing characterization in batch reactors using the radiotracer technique. J. Radioanal. Nucl. Chem. 1999, 241, 337–340. [Google Scholar] [CrossRef]
- Kaya, M. Current WEEE recycling solutions. In Waste Electrical and Electronic Equipment Recycling: Aqueous Recovery Methods; Woodhead Publishing: Sawston, UK, 2018; pp. 33–93. [Google Scholar] [CrossRef]
- Smolinski, T.; Rogowski, M.; Brykala, M.; Pyszynska, M.; Chmielewski, A.G. Studies on hydrometallurgical processes using nuclear techniques to be applied in copper industry. I. Application of 64Cu radiotracer for investigation of copper ore leaching. Nukleonika 2018, 63, 123–129. [Google Scholar] [CrossRef]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end of life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Panigrahi, M.; Grabda, M.; Kozak, D.; Dorai, A.; Shibata, E.; Kawamura, J.; Nakamura, T. Liquid–liquid extraction of neodymium ions from aqueous solutions of NdCl3 by phosphonium-based ionic liquids. Sep. Purif. Technol. 2016, 171, 263–269. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Smolders, S.; Li, X.; Raiguel, S.; Nies, E.; de Vos, D.E.; Binnemans, K. Enhancing Metal Separations by Liquid–Liquid Extraction Using Polar Solvents. Chem. -A Eur. J. 2019, 25, 9197–9201. [Google Scholar] [CrossRef]
- Gloe, K.; Mühl, P. Determination of Metal Extraction Process Parameters Using Tracer Technique. Isot. Isot. Environ. Health Stud. 1983, 19, 257–260. [Google Scholar] [CrossRef]
- Sarkar, R.; Dey, P.; Basu, S. Radiotracer study of synergic effect of trioctyl phosphine oxide on the solvent extraction of gold (III)-β-diketonates. J. Radioanal. Nucl. Chem. 2012, 292, 131–139. [Google Scholar] [CrossRef]
- Dey, P.; Basu, S. Radiotracer study of the effects of organophosphorus donors on the extraction of iron(III) with 2-hydroxy-N-phenylbenzamide in n-butanol. Radiochemistry 2011, 53, 370–374. [Google Scholar] [CrossRef]
- Parent, M.; Cornelis, R.; Dams, R.; Alt, F. Investigation of extraction and back-extraction behaviour of platinum (IV) with rubeanic acid in tributyl phosphate, with tributyl phosphate and with thenoyltrifuoroacetone in n-butyl alcohol-acetophenone by means of platinum-191 radiotracer for platinum-enrichment purposes. Anal. Chim. Acta 1993, 281, 153–160. [Google Scholar] [CrossRef]
- Rotuska, K.; Chmielewski, T. Growing role of solvent extraction in copper ores processing. Physicochem. Probl. Miner. Process. 2008, 42, 29–36. [Google Scholar]
- Chmielewski, T. Hydrometallurgy in Kghm Polska Miedz SA—Circumstances, Needs and Perspectives of Application. Sep. Sci. Technol. 2012, 47, 1264–1277. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar] [CrossRef]
- Tunsu, C.; Lapp, J.B.; Ekberg, C.; Retegan, T. Selective separation of yttrium and europium using Cyanex 572 for applications in fluorescent lamp waste processing. Hydrometallurgy 2016, 166, 98–106. [Google Scholar] [CrossRef]
- Ciacci, L.; Vassura, I.; Passarini, F. Shedding Light on the Anthropogenic Europium Cycle in the EU–28. Marking Product Turnover and Energy Progress in the Lighting Sector. Resources 2018, 7, 59. [Google Scholar] [CrossRef]
- Preston, J.S.; Preez, A.C.D. The solvent extraction of europium (II) by some organophosphorus and carboxylic acids. Solvent Extr. Ion Exch. 1991, 9, 237–257. [Google Scholar] [CrossRef]
- Saad, E.A.; El-Atrash, A.M. Thermodynamics of the extraction of europium (III) radiotracer by benzyl-phosphonic acid monoester. J. Radioanal. Nucl. Chem. Artic. 1989, 134, 415–421. [Google Scholar] [CrossRef]
- Ghani, L.; Shahida, S.; Ali, A.; Khan, M.H.; Aziz, B.; Masood, M.; Badshah, S.L.; Khan, M. Liquid–liquid extraction of Eu(lll) using synergic mixture of 1-phenyl-3-methyl-4-trifluoroacetyl-2-pyrazolin-5-one and crown ethers. SN Appl. Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological Evaluations of Rare Earths and Their Health Impacts to Workers: A Literature Review. Saf. Health Work. 2013, 4, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Luo, Z. A Review on X-ray Excited Emission Decay Dynamics in Inorganic Scintillator Materials. Photonics 2021, 8, 71. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, H.; Smart, N.G.; Wai, C.M. Studies on in-situ chelation/supercritical fluid extraction of lanthanides and actinides using a radiotracer technique. Sep. Sci. Technol. 2001, 36, 1149–1162. [Google Scholar] [CrossRef]
- Regueiro, M.; Alonso-Jimenez, A. Minerals in the future of Europe. Miner. Econ. 2021, 34, 209–224. [Google Scholar] [CrossRef]
- Schmidt, M. Scarcity and Environmental Impact of Mineral Resources—An Old and Never-Ending Discussion. Resources 2019, 8, 2. [Google Scholar] [CrossRef]
- Burlakovs, J.; Vincevica-Gaile, Z.; Krievans, M.; Jani, Y.; Horttanainen, M.; Pehme, K.M.; Dace, E.; Setyobudi, R.H.; Pilecka, J.; Denafas, G.; et al. Platinum Group Elements in Geosphere and Anthroposphere: Interplay among the Global Reserves, Urban Ores, Markets and Circular Economy. Minerals 2020, 10, 558. [Google Scholar] [CrossRef]
- Hughes, A.E.; Haque, N.; Northey, S.A.; Giddey, S. Platinum Group Metals: A Review of Resources, Production and Usage with a Focus on Catalysts. Resources 2021, 10, 93. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Y.; Zhou, Y.; Zhang, B.; Liu, G.; Li, Q.; Yang, Y.; Jiang, T. A Review of Recovery of Palladium from the Spent Automobile Catalysts. Metals 2022, 12, 533. [Google Scholar] [CrossRef]
- Eccles, S.F. Gamma ray spectroscopy of 107Cd, 109Pd and 111Pd. Physica 1962, 28, 251–261. [Google Scholar] [CrossRef]
- Nichols, A.L. Comments on evaluation Pd-Comments on evaluation of decay data A.1. Eval. Proced. 2009, pp. 1–10. Available online: http://www.lnhb.fr/nuclides/Pd-109_com.pdf (accessed on 10 March 2022).
- Jiang, J.; Liu, C.; Zhou, W.; Gao, H. The extraction of low-concentrations of gold(I) with 198Au as a radiotracer. J. Radioanal. Nucl. Chem. 2002, 254, 405–408. [Google Scholar] [CrossRef]
- Tom, H.; Henrik, S.; Kari, A.A. Minerals for The Green Economy. Trondheim, Norway. 2017, pp. 1–15. Available online: https://www.ngu.no/sites/default/files/Minerals%20for%20the%20green%20economy%202016_screen.pdf (accessed on 3 February 2022).
- Hagelüken, C.; Goldmann, D. Recycling and circular economy—Towards a closed loop for metals in emerging clean technologies. Miner. Econ. 2022, 1, 1–24. [Google Scholar] [CrossRef]
- Billah, A.A.; Pujiyanto, A.; Hambali, H.; Lestari, E. Recovery of Gold from Radioactive Gold Waste Using the Redox Replacement Method with Zn-Foil Reductor. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 022002. [Google Scholar] [CrossRef]
- Dey, P.; Banerjee, S.; Basu, S. Radiotracer study of synergistic effects of neutral donors on the extraction of gold with N-(Thioacetyl)benzamide in chloroform. Radiochemistry 2012, 54, 153–158. [Google Scholar] [CrossRef]
- Jiang, J.; Zhou, W.; Gao, H.; Wu, J.; Xu, G. Solvent extraction and stripping of gold (I) cyanide in the tetradecyldimethylbenzylammonium chloride system. Hydrometallurgy 2003, 70, 73–81. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Zhou, W.; Gao, H.; Wu, J. Extraction of gold from alkaline cyanide solution by the tetradecyldimethylbenzylammonium chloride/tri-n-butyl phosphate/n-heptane system based on a. Phys. Chem. Chem. Phys. 2002, 4, 4489–4494. Available online: https://pubs.rsc.org/en/content/articlehtml/2002/cp/b203467k (accessed on 1 December 2022). [CrossRef]
- Mandal, M.; Sarkar, R.; Basu, S. Determination of solubility parameters of cobalt (II) and indium (III) oxinates by tracer technique. Radiochim. Acta 2009, 97, 759–762. [Google Scholar] [CrossRef]
- Wang, K.; Pei, P.; Zuo, Y.; Wei, M.; Wang, H.; Zhang, P.; Chen, Z.; Shang, N. Magnetic zinc-air batteries for storing wind and solar energy. IScience 2022, 25, 103837. [Google Scholar] [CrossRef]
- Lerum, H.V.; Sand, S.; Eriksen, D.Ø.; Wibetoe, G.; Omtvedt, J.P. Comparison of single-phase and two-phase measurements in extraction, separation and back-extraction of Cd, Zn and Co from a multi-element matrix using Aliquat 336. J. Radioanal. Nucl. Chem. 2020, 324, 1203–1214. [Google Scholar] [CrossRef]
- Sokolov, A.; Valeev, D.; Kasikov, A. Solvent extraction of iron (III) from Al chloride solution of bauxite HCl leaching by mixture of aliphatic alcohol and ketone. Metals 2021, 11, 321. [Google Scholar] [CrossRef]
- Grimsley, L.F. Iron and Cobalt. Patty’s Toxicol. 2021, 647. [Google Scholar] [CrossRef]
- Mishra, S.P.; Singh, V.K. Radiotracer technique in adsorption study—XIII. Adsorption of barium and strontium ions on chromium (IV) oxide powder. Appl. Radiat. Isot. 1995, 46, 847–853. [Google Scholar] [CrossRef]
- Gujar, R.B.; Mohapatra, P.K.; Iqbal, M.; Huskens, J.; Verboom, W. Selective uptake of thorium(IV) from nitric acid medium using two extraction chromatographic resins based on diglycolamide-calix[4]arenes: Application to thorium-uranyl separation in an actual sample. J. Chromatogr. A 2021, 1653, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baybaş, D.; Ulusoy, U. Polyacrylamide-clinoptilolite/Y-zeolite composites: Characterization and adsorptive features for terbium. J. Hazard. Mater. 2011, 187, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.S.; Battula, R. Removal of cerium ions from aqueous solution by hydrous ferric oxide–A radiotracer study. J. Hazard. Mater. 2011, 186, 1028–1032. Available online: https://www.sciencedirect.com/science/article/pii/S0304389410015116 (accessed on 29 September 2022). [CrossRef] [PubMed]
- Johnson, B.E.; Santschi, P.H.; Chuang, C.Y.; Otosaka, S.; Addleman, R.S.; Douglas, M.; Rutledge, R.D.; Chouyyok, W.; Davidson, J.D.; Fryxell, G.E.; et al. Collection of lanthanides and actinides from natural waters with conventional and nanoporous sorbents. Environ. Sci. Technol. 2012, 46, 11251–11258. [Google Scholar] [CrossRef]
- Bertelsen, E.; Deodhar, G.; Kluherz, K.T.; Davidson, M.; Adams, M.L.; Trewyn, B.G.; Shafer, J.C. Microcolumn lanthanide separation using bis-(2-ethylhexyl) phosphoric acid functionalized ordered mesoporous carbon materials. J. Chromatogr. A 2019, 1595, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Symeopoulos, B.; Soupioni, M.; Misaelides, P.; Godelitsas, A.; Barbayiannis, N. Neodymium sorption by clay minerals and zeoliferous rocks. J. Radioanal. Nucl. Chem. 1996, 212, 421–429. [Google Scholar] [CrossRef]
- Xiangke, W.; Wenming, D.; Xiongxin, D.; Aixia, W.; Jinzhou, D.; Zuyi, T. Sorption and desorption of Eu and Yb on alumina: Mechanisms and effect of fulvic acid. Appl. Radiat. Isot. 2000, 52, 165–173. [Google Scholar] [CrossRef]
- Ansari, S.A.; Mohapatra, P.K.; Iqbal, M.; Huskens, J.; Verboom, W. Sorption of americium(III) and europium(III) from nitric acid solutions by a novel diglycolamide-grafted silica-based resins: Part 2. Sorption isotherms, column and radiolytic stability studies. Radiochim. Acta 2014, 102, 903–910. [Google Scholar] [CrossRef]
- Koeppenkastrop, D.; de Carlo, E.H. Uptake of Rare Earth Elements from Solution by Metal Oxides. Environ. Sci. Technol. 1993, 27, 1796–1802. [Google Scholar] [CrossRef]
- Snow, M.; Ward, J. Fundamental distribution coefficient data and separations using eichrom extraction chromatographic resins. J. Chromatogr. A 2020, 1620, 460833. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.; Horwitz, E.P.; Sajdak, L.R.; Renato, C. An improved extraction chromatographic resin for the separation of uranium from acidic nitrate media. Talanta 2001, 54, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Momen, M.A.; Healy, M.R.; Tsouris, C.; Jansone-Popova, S.; Depaoli, D.W.; Moyer, B.A. Extraction Chromatographic Materials for Clean Hydrometallurgical Separation of Rare-Earth Elements Using Diglycolamide Extractants. Ind. Eng. Chem. Res. 2019, 58, 20081–20089. [Google Scholar] [CrossRef]
- Momen, M.A.; Dietz, M.L. High-capacity extraction chromatographic materials based on polysulfone microcapsules for the separation and preconcentration of lanthanides from aqueous solution. Talanta 2019, 197, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.M.; Raieh, M.; El-Dessouky, M.; Aly, H.F. Extraction chromatography of some metal radiotracers using adogen-381 HCl and KSCN systems. Chromatographia 1982, 15, 315–317. [Google Scholar] [CrossRef]
- Devi, P.S.R.; Joshi, S.; Verma, R.; Reddy, A.V.R.; Lali, A.M.; Gantayet, L.M. Ion-exchange separation of 60Co and 125Sb from zirconium for radioactive waste management. Nucl. Technol. 2010, 171, 220–227. [Google Scholar] [CrossRef]
- Mandal, M.; Dhara, S.; Basu, S. Separation of Carrier-Free 115mIn from Its Parent 115Cd Using the Synthesized TODGA-Impregnated Silica Gel. Radiochemistry 2018, 60, 548–551. [Google Scholar] [CrossRef]
- Ng, Q.K.T.; Olariu, C.I.; Yaffee, M.; Taelman, V.F.; Marincek, N.; Krause, T.; Meier, L.; Walter, M.A. Indium-111 labeled gold nanoparticles for in-vivo molecular targeting. Biomaterials 2014, 35, 7050–7057. [Google Scholar] [CrossRef]
- Mandal, M.; Majumdar, D. Radiotracer studies for the uptake of silver from aqueous solution by TODGA-impregnated silica gel. Radiochemistry 2015, 57, 417–420. [Google Scholar] [CrossRef]
- Greenberg, R.R.; Bode, P.; de Nadai Fernandes, E.A. Neutron activation analysis: A primary method of measurement. Spectrochim. Acta Part B 2011, 66, 193–241. [Google Scholar] [CrossRef]
- Coler, D. The 5 Most Common Ways to Prepare Samples for XRF Analysis. Available online: https://www.armi.com/blog/the-5-most-common-ways-to-prepare-samples-for-xrf-analysis (accessed on 24 May 2017).
- Duchesne, J.; Belgica, G.B. XRF major and trace element determination in Fe-Ti oxide minerals. Orbi. Uliege. Be 2009, 12, 205–212. Available online: https://orbi.uliege.be/bitstream/2268/6194/1/VOL%2012%20PART%203-4%20DUCHESNE%20205-212.pdf (accessed on 1 December 2022).







| Metal Radiotracers Available | Eluted Radiotracer | ||
|---|---|---|---|
| Cu2+, Fe3+, Zn2+, Hg2+ | 3.75 cm3 | 0.1M HCl | Fe3+ |
| 9.87 cm3 | 4.0 KSCN | Cu2+ | |
| 9.50 cm3 | 8.0 M KSCN | Hg2+ | |
| 2.50 cm3 | 1.0 M H4CH3COO | Zn2+ | |
| Sc3+, Fe3+, Hg2+, Cd2+ | 2.62 cm3 | 8.0 M HCl | Sc3+ |
| 3.75 cm3 | 0.1 M HCl | Fe3+ | |
| 9.38 cm3 | 8.0 M KSCN | Hg2+ | |
| 3.75 cm3 | 8.0 M HNO3 | Cd2+ | |
| Co2+, Cu2+, Zn2+ | 3.13 cm3 | 4.0 M HCl | Co2+ |
| 9.37 cm3 | 4.0 M KSCN | Cu2+ | |
| 3.12 cm3 | 1.0 M H4CH3COO | Zn2+ | |
| Co2+, Cu2+, Cd2+ | 5.62 cm3 | 0.01 M HCl | Co2+ |
| 9.37 cm3 | 4.0 M KSCN | Cu2+ | |
| 3.75 cm3 | 8.0 M HNO3 | Cd2+ | |
| Cu2+, Hg2+, Cd2+ | 9.50 cm3 | 4.0 M KSCN | Cu2+ |
| 9.25 cm3 | 8.0 M KSCN | Hg2+ | |
| 7.75 cm3 | 8.0 M HNO3 | Cd2+ | |
| Sc3+, Ce3+, Eu3+, Tm3+, Cu2+, Zn2+ | 2.63 cm3 | 0.1 M HCl | Sc3+, Ce3+, Eu3+, Tm3+ |
| 9.63 cm3 | 4.0 M KSCN | Cu2+ | |
| 3.70 cm3 | 1.0 M NH4CH3COO | Zn2+ | |
| Sc3+, Ce3+, Eu3+, Tm3+, Mo6+ | 2.25 cm3 | 0.1 M HCl | Sc3+, Ce3+, Eu3+, Tm3+ |
| 2.0 cm3 | 4.0 M HNO3 | Mo6+ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiprono, N.R.; Smolinski, T.; Rogowski, M.; Chmielewski, A.G. The State of Critical and Strategic Metals Recovery and the Role of Nuclear Techniques in the Separation Technologies Development: Review. Separations 2023, 10, 112. https://doi.org/10.3390/separations10020112
Kiprono NR, Smolinski T, Rogowski M, Chmielewski AG. The State of Critical and Strategic Metals Recovery and the Role of Nuclear Techniques in the Separation Technologies Development: Review. Separations. 2023; 10(2):112. https://doi.org/10.3390/separations10020112
Chicago/Turabian StyleKiprono, Nelson R., Tomasz Smolinski, Marcin Rogowski, and Andrzej G. Chmielewski. 2023. "The State of Critical and Strategic Metals Recovery and the Role of Nuclear Techniques in the Separation Technologies Development: Review" Separations 10, no. 2: 112. https://doi.org/10.3390/separations10020112
APA StyleKiprono, N. R., Smolinski, T., Rogowski, M., & Chmielewski, A. G. (2023). The State of Critical and Strategic Metals Recovery and the Role of Nuclear Techniques in the Separation Technologies Development: Review. Separations, 10(2), 112. https://doi.org/10.3390/separations10020112