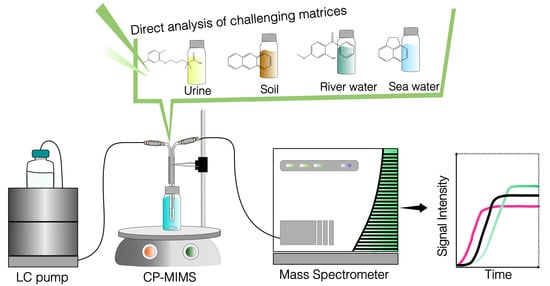
Condensed Phase Membrane Introduction Mass Spectrometry: A Direct Alternative to Fully Exploit the Mass Spectrometry Potential in Environmental Sample Analysis
Abstract
:1. Introduction
- Real-time results: there is a negligible delay between the sampling and analysis. This is a key factor for process monitoring and space mapping in environmental applications [25].
- Preconcentration factor: depending on the operating parameters, CP-MIMS can provide significant enrichments with a consequent boost in sensitivity and limit of detection (LOD) [26]. For most online monitoring applications there is no alternative technique with similar performance. Moreover, the use of a divert valve in the system permits to operate in static AP mode in the membrane (stopped-flow mode). During selected intervals of time, the AP is stopped and allowed to equilibrate with the membrane interface, leading to higher analytical sensitivity [20,21,23,26].
- Flexibility: by combining different types of membranes, acceptor phases, or ionization methods it is possible to adjust parameters accordingly to different types of analytes and matrices. Samples eligible for CP-MIMS can be both homogeneous and heterogeneous liquids: membrane can also be functionalized (e.g., immobilizing enzymes or functional groups) to make reactions take place before permeation [27].
- Automatability: theoretically, no further operation is required more than the establishment of membrane–sample contact; this operation can be included in autosampler or in analytical procedures [20].
- Cost Effectiveness: the use of CP-MIMS eliminates the need for specific sample preparation devices, disposable consumables, and chromatographic instruments. In addition, the system itself is simple and reusable [12].
- Thus, CP-MIMS is a promising technique for real-time monitoring applications, especially in the environmental and pharmaceutical fields [20,31,32,33,34,35,36]. This review aims to introduce CP-MIMS theoretical fundamentals and basic functioning principles and to survey its recent and contemporary applications, focusing on those applications which include the immersion probe sampling interface variant.
2. CP-MIMS Fundamentals
3. Suitable Analytes
4. Applications
4.1. Rapid Detection of Contaminants in Challenging Matrices
4.2. On-Line Monitoring
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP | Acceptor Phase |
| APCI | Atmospheric Pressure Chemical Ionization |
| API | Atmospheric Pressure Ionization |
| APPI | Atmospheric Pressure Photo Ionization |
| AMS | Ambient Mass Spectrometry |
| CI | Chemical Ionization |
| CP-MIMS | Condensed Phase-Membrane Introduction Mass spectrometry |
| DEI | Direct Electron Ionization |
| EI | Electron Ionization |
| ESI | Electrospray Ionization |
| LC | Liquid Chromatography |
| LEI | Liquid Electron Ionization |
| MEPS | Micro Extraction by Packed Sorbent |
| MIMS | Membrane Introduction Mass Spectrometry |
| MS | Mass Spectrometry |
| MS/MS | Tandem Mass Spectrometry |
| NAs | Naphthenic Acids |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PCI | Positive Chemical Ionization |
| SPME | Solid Phase Micro Extraction |
| SBSE | Stir Bar Sorptive Extraction |
| SRM | Selected Reaction Monitoring |
References
- Hoch, G.; Kok, B. A mass spectrometer inlet system for sampling gases dissolved in liquid phases. Arch. Biochem. Biophys. 1963, 101, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Krogh, E.T.; Gill, C.G. Condensed Phase Membrane Introduction Mass Spectrometry—Continuous, Direct and Online Measurements in Complex Samples; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 79. [Google Scholar]
- Tarkiainen, V.; Kotiaho, T.; Mattila, I.; Virkajärvi, I.; Aristidou, A.; Ketola, R.A. On-line monitoring of continuous beer fermentation process using automatic membrane inlet mass spectrometric system. Talanta 2005, 65, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Bastidas-Oyanedel, J.-R.; Mohd-Zaki, Z.; Pratt, S.; Steyer, J.-P.; Batstone, D.J. Development of membrane inlet mass spectrometry for examination of fermentation processes. Talanta 2010, 83, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Namieśnik, J. Trends in Environmental Analytics and Monitoring. Crit. Rev. Anal. Chem. 2000, 30, 221–269. [Google Scholar] [CrossRef]
- Termopoli, V.; Piergiovanni, M.; Cappiello, A.; Palma, P.; Famiglini, G. Tyrosol and Hydroxytyrosol Determination in Extra Virgin Olive Oil with Direct Liquid Electron Ionization-Tandem Mass Spectrometry. Separations 2021, 8, 173. [Google Scholar] [CrossRef]
- Saurina, J. Flow-injection analysis for multi-component determinations of drugs based on chemometric approaches. TrAC Trends Anal. Chem. 2010, 29, 1027–1037. [Google Scholar] [CrossRef]
- Tzanavaras, P.D.; Themelis, D.G. Review of recent applications of flow injection spectrophotometry to pharmaceutical analysis. Anal. Chim. Acta 2007, 588, 1–9. [Google Scholar] [CrossRef]
- Lemos, V.A.; Oliveira, R.V.; Lopes dos Santos, W.N.; Menezes, R.M.; Santos, L.B.; Costa Ferreira, S.L. Liquid phase microextraction associated with flow injection systems for the spectrometric determination of trace elements. TrAC Trends Anal. Chem. 2019, 110, 357–366. [Google Scholar] [CrossRef]
- Mansour, F.R.; Danielson, N.D. Reverse flow-injection analysis. TrAC Trends Anal. Chem. 2012, 40, 1–14. [Google Scholar] [CrossRef]
- Davey, N.G.; Krogh, E.T.; Gill, C.G. Membrane-introduction mass spectrometry (MIMS). TrAC Trends Anal. Chem. 2011, 30, 1477–1485. [Google Scholar] [CrossRef]
- Krogh, E.T.; Gill, C.G. Membrane introduction mass spectrometry (MIMS): A versatile tool for direct, real-time chemical measurements. J. Mass Spectrom. 2014, 49, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Kotiaho, T.; Lauritsen, F.R.; Choudhury, T.K.; Cooks, R.G.; Tsao, G.T. Membrane Introduction Mass Spectrometry. Anal. Chem. 1991, 63, 875A–883A. [Google Scholar] [CrossRef]
- Johnson, R.C.; Cooks, R.G.; Allen, T.M.; Cisper, M.E.; Hemberger, P.H. Membrane introduction Mass Spectrometry: Trends and applications. Mass Spectrom. Rev. 2000, 19, 1–37. [Google Scholar] [CrossRef]
- Cisper, M.E.; Gill, C.G.; Townsend, L.E.; Hemberger, P.H. Online Detection of Volatile Organic Compounds in Air at Parts-per-Trillion Levels by Membrane Introduction Mass Spectrometry. Anal. Chem. 1995, 67, 1413–1417. [Google Scholar] [CrossRef]
- Ketola, R.A.; Kotiaho, T.; Cisper, M.E.; Allen, T.M. Environmental applications of membrane introduction mass spectrometry. J. Mass Spectrom. 2002, 37, 457–476. [Google Scholar] [CrossRef]
- Tsamba, L.; Correc, O.; Le Cloirec, P.; Cimetière, N. Analysis of chlorination by-products in swimming pool water by membrane introduction mass spectrometry—Influence of water physicochemical parameters. Rapid Commun. Mass Spectrom. 2019, 33, 710–718. [Google Scholar] [CrossRef]
- Cherebillo, V.Y.; Elizarov, A.Y.; Polegaev, A.V. Membrane-Introduction Mass Spectrometry Analysis of Desflurane, Propofol and Fentanyl in Plasma and Cerebrospinal Fluid for Estimation BBB Properties. Exp. Neurobiol. 2015, 24, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Giannoukos, S.; Brkić, B.; Taylor, S.; France, N. Membrane inlet mass spectrometry for Homeland security and forensic applications. J. Am. Soc. Mass Spectrom. 2015, 26, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Duncan, K.D.; Willis, M.D.; Krogh, E.T.; Gill, C.G. A miniature condensed-phase membrane introduction mass spectrometry (CP-MIMS) probe for direct and on-line measurements of pharmaceuticals and contaminants in small, complex samples. Rapid Commun. Mass Spectrom. 2013, 27, 1213–1221. [Google Scholar] [CrossRef]
- Duncan, K.D.; McCauley, E.P.B.; Krogh, E.T.; Gill, C.G. Characterization of a condensed-phase membrane introduction mass spectrometry (CP-MIMS) interface using a methanol acceptor phase coupled with electrospray ionization for the continuous on-line quantitation of polar, low-volatility analytes at trace level. Rapid Commun. Mass Spectrom. 2011, 25, 1141–1151. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Zampieri, D.; Gennaro, M.C. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.D.; Vandergrift, G.W.; Krogh, E.T.; Gill, C.G. Ionization suppression effects with condensed phase membrane introduction mass spectrometry: Methods to increase the linear dynamic range and sensitivity. J. Mass Spectrom. 2014, 50, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Schneditz, D.; Sauseng, N. Diffusive solute transport in hollow fiber dialyzers is not affected by variable feed viscosity. Biocybern. Biomed. Eng. 2022, 42, 1112–1122. [Google Scholar] [CrossRef]
- Termopoli, V.; Torrisi, E.; Famiglini, G.; Palma, P.; Zappia, G.; Cappiello, A.; Vandergrift, G.W.; Zvekic, M.; Krogh, E.T.; Gill, C.G. Mass Spectrometry Based Approach for Organic Synthesis Monitoring. Anal. Chem. 2019, 91, 11916–11922. [Google Scholar] [CrossRef]
- Willis, M.D.; Duncan, K.D.; Krogh, E.T.; Gill, C.G. Delicate polydimethylsiloxane hollow fibre membrane interfaces for condensed phase membrane introduction mass spectrometry (CP-MIMS). Rapid Commun. Mass Spectrom. 2014, 28, 671–681. [Google Scholar] [CrossRef]
- Creba, A.S.; Weissfloch, A.N.E.; Krogh, E.T.; Gill, C.G. An enzyme derivatized polydimethylsiloxane (PDMS) membrane for use in membrane introduction mass spectrometry (MIMS). J. Am. Soc. Mass Spectrom. 2007, 18, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. TrAC Trends Anal. Chem. 2015, 71, 2–8. [Google Scholar] [CrossRef]
- Armenta, S.; Esteve-Turrillas, F.A.; Garrigues, S.; de la Guardia, M. 2.34—Green Analytical Chemistry. In Comprehensive Foodomics; Cifuentes, A., Ed.; Elsevier: Oxford, UK, 2021; pp. 483–493. ISBN 978-0-12-816396-2. [Google Scholar]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Monaghan, J.; Xin, Q.; Aplin, R.; Jaeger, A.; Heshka, N.E.; Hounjet, L.J.; Gill, C.G.; Krogh, E.T. Aqueous naphthenic acids and polycyclic aromatic hydrocarbons in a meso-scale spill tank affected by diluted bitumen analyzed directly by membrane introduction mass spectrometry. J. Hazard. Mater. 2022, 440, 129798. [Google Scholar] [CrossRef]
- Monaghan, J.; Richards, L.C.; Vandergrift, G.W.; Hounjet, L.J.; Stoyanov, S.R.; Gill, C.G.; Krogh, E.T. Direct mass spectrometric analysis of naphthenic acids and polycyclic aromatic hydrocarbons in waters impacted by diluted bitumen and conventional crude oil. Sci. Total Environ. 2021, 765, 144206. [Google Scholar] [CrossRef]
- Duncan, K.D.; Richards, L.C.; Monaghan, J.; Simair, M.C.; Ajaero, C.; Peru, K.M.; Friesen, V.; McMartin, D.W.; Headley, J.V.; Gill, C.G.; et al. Direct analysis of naphthenic acids in constructed wetland samples by condensed phase membrane introduction mass spectrometry. Sci. Total Environ. 2020, 716, 137063. [Google Scholar] [CrossRef]
- Vandergrift, G.W.; Monaghan, J.; Krogh, E.T.; Gill, C.G. Direct Analysis of Polyaromatic Hydrocarbons in Soil and Aqueous Samples Using Condensed Phase Membrane Introduction Tandem Mass Spectrometry with Low-Energy Liquid Electron Ionization. Anal. Chem. 2019, 91, 1587–1594. [Google Scholar] [CrossRef]
- Vandergrift, G.W.; Krogh, E.T.; Gill, C.G. Direct, Isomer-Specific Quantitation of Polycyclic Aromatic Hydrocarbons in Soils Using Membrane Introduction Mass Spectrometry and Chemical Ionization. Anal. Chem. 2020, 92, 15480–15488. [Google Scholar] [CrossRef]
- Vandergrift, G.W.; Lattanzio-Battle, W.; Rodgers, T.R.; Atkinson, J.B.; Krogh, E.T.; Gill, C.G. Geospatial Assessment of Trace-Level Benzophenone-3 in a Fish-Bearing River Using Direct Mass Spectrometry. ACS EST Water 2022, 2, 262–267. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2009; ISBN 978-0-521-87121-1. [Google Scholar]
- Cao, X.; Qiu, L.; Feng, X. Permeability, solubility, and diffusivity of aniline in poly(ether-b-amide) membranes pertaining to aniline removal from aqueous solutions by pervaporation and sorption. J. Membr. Sci. 2022, 642, 120006. [Google Scholar] [CrossRef]
- LaPack, M.A.; Tou, J.C.; Enke, C.G. Membrane mass spectrometry for the direct trace analysis of volatile organic compounds in air and water. Anal. Chem. 1990, 62, 1265–1271. [Google Scholar] [CrossRef]
- VanHassel, E.; Bier, M.E. An electrospray membrane probe for the analysis of volatile and semi-volatile organic compounds in water. Rapid Commun. Mass Spectrom. 2007, 21, 413–420. [Google Scholar] [CrossRef]
- Vandergrift, G.W.; Krogh, E.T.; Gill, C.G. Polymer Inclusion Membranes with Condensed Phase Membrane Introduction Mass Spectrometry (CP-MIMS): Improved Analytical Response Time and Sensitivity. Anal. Chem. 2017, 89, 5629–5636. [Google Scholar] [CrossRef]
- Termopoli, V.; Famiglini, G.; Palma, P.; Cappiello, A.; Vandergrift, G.W.; Krogh, E.T.; Gill, C.G. Condensed Phase Membrane Introduction Mass Spectrometry with Direct Electron Ionization: On-line Measurement of PAHs in Complex Aqueous Samples. J. Am. Soc. Mass Spectrom. 2016, 27, 301–308. [Google Scholar] [CrossRef]
- Duncan, K.D.; Letourneau, D.R.; Vandergrift, G.W.; Jobst, K.; Reiner, E.; Gill, C.G.; Krogh, E.T. A semi-quantitative approach for the rapid screening and mass profiling of naphthenic acids directly in contaminated aqueous samples. J. Mass Spectrom. 2016, 51, 44–52. [Google Scholar] [CrossRef]
- Termopoli, V.; Famiglini, G.; Palma, P.; Piergiovanni, M.; Cappiello, A. Atmospheric Pressure Vaporization Mechanism for Coupling a Liquid Phase with Electron Ionization Mass Spectrometry. Anal. Chem. 2017, 89, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Termopoli, V.; Famiglini, G.; Palma, P.; Piergiovanni, M.; Rocio-Bautista, P.; Ottaviani, M.F.; Cappiello, A.; Saeed, M.; Perry, S. Evaluation of a liquid electron ionization liquid chromatography–mass spectrometry interface. J. Chromatogr. A 2019, 1591, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Famiglini, G.; Palma, P.; Termopoli, V.; Cappiello, A.; Tsizin, S.; Seemann, B.; Alon, T.; Fialkov, A.B.; Amirav, A. Electron Ionization LC-MS. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 79, pp. 1–28. ISBN 978-0-444-63914-1. [Google Scholar]
- Bianchi, F.; Ilag, L.; Termopoli, V.; Mendez, L. Advances in MS-Based Analytical Methods: Innovations and Future Trends. J. Anal. Methods Chem. 2018, 2018, 2084567. [Google Scholar] [CrossRef] [PubMed]
- Feehan, J.F.; Monaghan, J.; Gill, C.G.; Krogh, E.T. Direct Measurement of Acid Dissociation Constants of Trace Organic Compounds at Nanomolar Levels in Aqueous Solution by Condensed Phase–Membrane Introduction Mass Spectrometry. Environ. Toxicol. Chem. 2019, 38, 1879–1889. [Google Scholar] [CrossRef]
- Duncan, K.D.; Volmer, D.A.; Gill, C.G.; Krogh, E.T. Rapid Screening of Carboxylic Acids from Waste and Surface Waters by ESI-MS/MS Using Barium Ion Chemistry and On-Line Membrane Sampling. J. Am. Soc. Mass Spectrom. 2016, 27, 443–450. [Google Scholar] [CrossRef]
- Vandergrift, G.W.; Lattanzio-Battle, W.; Krogh, E.T.; Gill, C.G. Condensed Phase Membrane Introduction Mass Spectrometry with In Situ Liquid Reagent Chemical Ionization in a Liquid Electron Ionization Source (CP-MIMS-LEI/CI). J. Am. Soc. Mass Spectrom. 2020, 31, 908–916. [Google Scholar] [CrossRef]
- Borden, S.A.; Damer, H.N.; Krogh, E.T.; Gill, C.G. Direct quantitation and characterization of fatty acids in salmon tissue by condensed phase membrane introduction mass spectrometry (CP-MIMS) using a modified donor phase. Anal. Bioanal. Chem. 2019, 411, 291–303. [Google Scholar] [CrossRef]
- Ghislain, T.; Faure, P.; Michels, R. Detection and Monitoring of PAH and Oxy-PAHs by High Resolution Mass Spectrometry: Comparison of ESI, APCI and APPI Source Detection. J. Am. Soc. Mass Spectrom. 2012, 23, 530–536. [Google Scholar] [CrossRef] [Green Version]
- De Melo, A.P.Z.; Hoff, R.B.; Molognoni, L.; Kleemann, C.R.; de Oliveira, T.; de Oliveira, L.V.A.; Daguer, H.; Barreto, P.L.M. Determination of Polycyclic Aromatic Hydrocarbons in Seafood by PLE-LC-APCI-MS/MS and Preliminary Risk Assessment of the Northeast Brazil Oil Spill. Food Anal. Methods 2022, 15, 1826–1842. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, K.; He, J.; Li, N.; You, H.; Jiang, J. Droplet spray ionization mass spectrometry for real-time monitoring of activation of peroxymonosulfate by 1,4-benzoquinone. Microchem. J. 2018, 139, 437–442. [Google Scholar] [CrossRef]
- Cruz, I.A.; Andrade, L.R.S.; Bharagava, R.N.; Nadda, A.K.; Bilal, M.; Figueiredo, R.T.; Ferreira, L.F.R. An overview of process monitoring for anaerobic digestion. Biosyst. Eng. 2021, 207, 106–119. [Google Scholar] [CrossRef]
- Liu, N.; Lu, X.; Yang, Y.; Yao, C.X.; Ning, B.; He, D.; He, L.; Ouyang, J. Monitoring binding affinity between drug and α1-acid glycoprotein in real time by Venturi easy ambient sonic-spray ionization mass spectrometry. Talanta 2015, 143, 240–244. [Google Scholar] [CrossRef]
- Domokos, A.; Madarász, L.; Stoffán, G.; Tacsi, K.; Galata, D.; Csorba, K.; Vass, P.; Nagy, Z.K.; Pataki, H. Real-Time Monitoring of Continuous Pharmaceutical Mixed Suspension Mixed Product Removal Crystallization Using Image Analysis. Org. Process Res. Dev. 2022, 26, 149–158. [Google Scholar] [CrossRef]
- Sun, J.; Yin, Y.; Li, W.; Jin, O.; Na, N. Chemical Reaction Monitoring by Ambient Mass Spectrometry. Mass Spectrom. Rev. 2022, 41, 70–99. [Google Scholar] [CrossRef]
- Nelson, J.H.L.; Friesen, D.A.; Gill, C.G.; Krogh, E.T. On-line measurement of oxidative degradation kinetics for trace gasoline contaminants in aqueous solutions and natural water by membrane introduction tandem mass spectrometry. J. Environ. Sci. Health Part A 2010, 45, 1720–1731. [Google Scholar] [CrossRef]
- Letourneau, D.R.; Gill, C.G.; Krogh, E.T. Photosensitized degradation kinetics of trace halogenated contaminants in natural waters using membrane introduction mass spectrometry as an in situ reaction monitor. Photochem. Photobiol. Sci. 2015, 14, 2108–2118. [Google Scholar] [CrossRef]
- Duncan, K.D.; Beach, D.G.; Wright, E.J.; Barsby, T.; Gill, C.G.; Krogh, E.T. Direct online quantitation of 2-methyl-3-methoxy-4-phenyl butanoic acid for total microcystin analysis by condensed phase membrane introduction tandem mass spectrometry. Anal. Methods 2018, 10, 3310–3316. [Google Scholar] [CrossRef]


| Analyte | CP-MIMS Variant | MS Interface * | Detection Limit Range (ng/mL) | Sample Matrix (Donor Phase) | Acceptor Phase | Acceptor Flow Rate (µL/min) | Ref. |
|---|---|---|---|---|---|---|---|
| Abietic acid, bromobenzoic acid, 2-chlorophenol, 2,4-dichlorophenol, 2,4,6-trichlorophenol, estrone, phenol, ethynyl estradiol, ibuprofen, nonylphenol, triclosan | Flow cell interface | ESI | 0.05–100 | Water | MeOH | 500 | [21] |
| Flow cell interface with stopped-flow mode | 0.005 for 2,4-dichlorophenol | ||||||
| Phenol, 2-chlorophenols, 2,4-dichlorophenols, 2,4,6-trichlorophenols, triclosan, gemfibrozil, nonylphenol | Flow cell interface | ESI | 0.05–2 | Drinking water, beer, urine, wastewater | MeOH | 500 | [20] |
| Miniature probe interface | 0.02–2 | 200 | |||||
| Aniline, aniline-d5, methylquinoline, | Immersion probe interface | APCI ESI | n/a | Wastewater urine | MeOH | 200 | [23] |
| Gemfibrozil | Immersion probe interface with stopped-flow mode | 0.005 | Deionized water | ||||
| Abietic acid, estrone, gemfibrozil, nonylphenol, 2,4,6-trichlorophenol, triclosan | Immersion probe interface | ESI | 0.6–3 | River and oil process wastewater | MeOH | 200 | [26] |
| Immersion probe interface with stopped-flow mode | 28x signal enhancement for gemfibrozil | ||||||
| PAHs (naphthalene, acenaphthene, fluorine, phenanthrene, anthracene, pyrene, chrysene, benzo[a]anthracene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno [1,2,3-cd]pyrene, benzo[g,h,i]perylene) | Immersion probe interface | DEI | n/a | River, sea water, hydrocarbon drilling rig production water | MeOH and ACN | 200 | [42] |
| Naphthenic acid isomer classes | Immersion probe interface | ESI | 5–10 (170 µm membrane thickness) | Oil sand process water, groundwater and seawater | MeOH | 200 | [43] |
| Phenyl-substituted carboxylic acids (2-phenylethanoic acid, 3-phenylpropanoic acid, 4- phenylbutanoic acid, lauric acid, gemfibrozil, cyclohexanebutytic acid, decanoic acid, perfluorodecanoic acid, bisphenol A, 1-octanol, nonylphenol, β-citronellol) | Immersion probe interface | ESI | 0.1–0.05 | Waste and surface water | MeOH | 200 | [49] |
| Gemfibrozil, nonylphenol, 2,4,6-trichlorophenol, Triclosan and naphthenic acids | Immersion probe interface (PIM) | ESI | 0.004–0.23 | Artificial urine, river and seawater | 0.046 mole fraction heptane/ MeOH | 75 | [41] |
| PAHs (naphthalene, anthracene, pyrene, benzo[a]pyrene, pyrene-d10) | Immersion probe interface | LEI | 61–330 (aqueous samples) 0.7–26 (mg/kg in soil samples) | Deionized water, seawater, river water, soil suspended in 2-propanol | MeOH/Heptane 85/15% v/v | 50 | [34] |
| Chlorobenzene, phenylacetylene, acetophenone, (R)-α-methyl benzylamine, ethyl bromoacetate, ethyl (R)-(1-phenylethyl)-glycinate, diethyl (R)-2,2-((1-phenylethyl)-azanediyl)-diacetate | Immersion probe interface | LEI | n/a - | Various organic-based polar solvents | 15:85 (v:v) Heptane/ MeOH | 100 | [25] |
| Linear saturated fatty acids (C12→22), eicosapentaenoic acid, docosahexaenoic acid | Immersion probe interface | ESI | 0.13–1.7 | Salmon tissue suspended in 50:50 (v:v) H2O:MeOH | MeOH | 75 | [51] |
| Various contaminants, pharmaceuticals, and naphthenic acids (aniline, 2-perfluorohexylethanoic, 4-tert-butylcyclohexanecarboxylic, decanoic and octanoic acid, gemfibrozil, ibuprofen, lauric acid, naproxen acid, perfluoro-n-octanoic acid, triclosan, 2-methyl-3-methoxy-4-phenyl butanoic acid, aniline-d5, 2-methoxylphenol, lauric acid-d2 | Immersion probe interface | ESI | n/a - | Water and heavy water | MeOH | 200 | [48] |
| Naphthenic acid isomer classes | Immersion probe interface | ESI | 20 | Oil sands process-affected waters | MeOH | 75 | [33] |
| Bis(2-ethylhexyl)-phthalate, dibutylphthalate, diethylphthalate | Immersion probe interface with stopped-flow mode | LEI/CI | 450 (Bis(2-ethylhexyl)-phthalate) | House dust in 2-propanol, ACN and deionized water | 70:30 (v:v) Acetonitrile and diethyl ether | 50 | [50] |
| PAHs (naphthalene, anthracene, phenanthrene, fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[a]pyrene, benzo[b]fluoranthene) | Immersion probe interface | LEI/CI | 100–1100 (ng/g) | Soil in dichloromethane or chloroform | 73:23 (v:v) dichloromethane/MeOH 87:13 (v:v) chloroform/MeOH | 10 | [35] |
| Naphthenic acids | Immersion probe interface | ESI | n/a | Bitumen and crude oil | MeOH/Heptane 85/15% (v/v) | 50 | [32] |
| PAHs (naphthalene, anthracene/phenanthrene, pyrene/fluoranthene) | Immersion probe interface with stopped-flow mode | LEI | 1–2 | ||||
| Benzophenone-3 | Modified immersion probe interface | LEI/CI | 0.02 | River water | MeOH/Heptane 85:15% (v/v) | 1 | [36] |
| Naphtenic acids | Immersion probe interface | ESI | n/a | Marine and fresh water | MeOH/Hexane 85/15% (v/v) | 50 | [31] |
| PAHs | LEI | 0.05–0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Termopoli, V.; Piergiovanni, M.; Ballabio, D.; Consonni, V.; Cruz Muñoz, E.; Gosetti, F. Condensed Phase Membrane Introduction Mass Spectrometry: A Direct Alternative to Fully Exploit the Mass Spectrometry Potential in Environmental Sample Analysis. Separations 2023, 10, 139. https://doi.org/10.3390/separations10020139
Termopoli V, Piergiovanni M, Ballabio D, Consonni V, Cruz Muñoz E, Gosetti F. Condensed Phase Membrane Introduction Mass Spectrometry: A Direct Alternative to Fully Exploit the Mass Spectrometry Potential in Environmental Sample Analysis. Separations. 2023; 10(2):139. https://doi.org/10.3390/separations10020139
Chicago/Turabian StyleTermopoli, Veronica, Maurizio Piergiovanni, Davide Ballabio, Viviana Consonni, Emmanuel Cruz Muñoz, and Fabio Gosetti. 2023. "Condensed Phase Membrane Introduction Mass Spectrometry: A Direct Alternative to Fully Exploit the Mass Spectrometry Potential in Environmental Sample Analysis" Separations 10, no. 2: 139. https://doi.org/10.3390/separations10020139
APA StyleTermopoli, V., Piergiovanni, M., Ballabio, D., Consonni, V., Cruz Muñoz, E., & Gosetti, F. (2023). Condensed Phase Membrane Introduction Mass Spectrometry: A Direct Alternative to Fully Exploit the Mass Spectrometry Potential in Environmental Sample Analysis. Separations, 10(2), 139. https://doi.org/10.3390/separations10020139