Health-Promoting Potential of Millet: A Review
Abstract
:1. Introduction
2. Methodology
3. Nutritional Quality of Millets
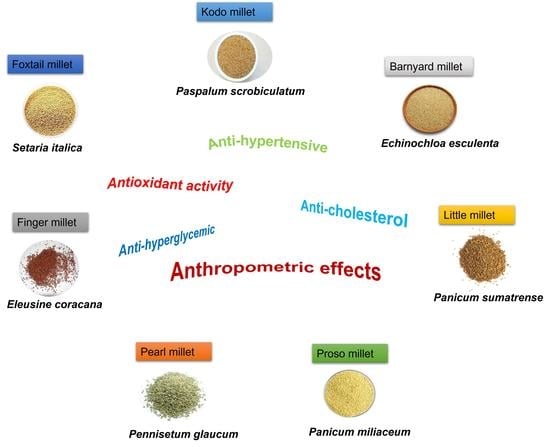
4. Beneficial Features of Millet
4.1. Antioxidant Activity
4.2. Anti-Hyperglycemic Effects
4.3. Anti-Cholesterol Effects
4.4. Anti-Hypertensive Effects
4.5. Anthropometric Effects
4.6. Effects on Gut Microbiota Composition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tripathi, M.K.; Jadam, R.S.; Kumar, A. Quality Management System in Millet and Sorghum. In Millets and Millet Technology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 363–379. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook; FAO: Rome, Italy, 2020. [Google Scholar]
- Saini, S.; Saxena, S.; Samtiya, M.; Puniya, M.; Dhewa, T. Potential of underutilized millets as Nutri-cereal: An overview. J. Food Sci. Technol. 2021, 58, 4465–4477. [Google Scholar] [CrossRef] [PubMed]
- Ertop, M.H.; Bektaş, M. Enhancement of bioavailable micronutrients and reduction of antinutrients in foods with some processes. Food Health 2018, 4, 159–165. [Google Scholar] [CrossRef]
- Yin, D.; Yin, X.; Wang, X.; Lei, Z.; Wang, M.; Guo, Y.; Aggrey, S.E.; Nie, W.; Yuan, J. Supplementation of amylase combined with glucoamylase or protease changes intestinal microbiota diversity and benefits for broilers fed a diet of newly harvested corn. J. Anim. Sci. Biotechnol. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Niranjan, K. Foxtail millet: Properties, processing, health benefits, and uses. Food Rev. Int. 2017, 34, 329–363. [Google Scholar] [CrossRef]
- Asrani, P.; Ali, A.; Tiwari, K. Millets as an alternative diet for gluten-sensitive individuals: A critical review on nutritional components, sensitivities and popularity of wheat and millets among consumers. Food Rev. Int. 2022, 38, 1–30. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; He, C.; Xue, P.; Zhang, L.; He, Z.; Zang, L.; Feng, B.; Sun, J.; Zheng, M. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol. Cancer 2020, 19, 46. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Chen, J.; Wang, C.; Molla, M.M.; Diao, X.; Shen, Q. In vitro starch digestibility, degree of gelatinization and estimated glycemic index of foxtail millet-derived products: Effect of freezing and frozen storage. J. Cereal Sci. 2016, 69, 166–173. [Google Scholar] [CrossRef]
- Akinola, S.A.; Badejo, A.A.; Osundahunsi, O.F.; Edema, M.O. Effect of preprocessing techniques on pearl millet flour and changes in technological properties. Int. J. Food Sci. Technol. 2017, 52, 992–999. [Google Scholar] [CrossRef]
- Ganguly, S.; Sabikhi, L.; Singh, A.K. Effect of whey-pearl millet-barley based probiotic beverage on Shigella-induced pathogenicity in murine model. J. Funct. Foods 2019, 54, 498–505. [Google Scholar] [CrossRef]
- Sarita; Singh, E. Potential of Millets: Nutrients Composition and Health Benefits. J. Sci. Innov. Res. 2016, 5, 46–50. [Google Scholar] [CrossRef]
- Srilekha, K.; Kamalaja, T.; Maheswari, K.U.; Rani, R.N. Nutritional Composition of Little Millet Flour. Int. Res. J. Pure Appl. Chem. 2019, 31, 1–4. [Google Scholar] [CrossRef]
- Tyl, C.; Marti, A.; Hayek, J.; Anderson, J.; Ismail, B.P. Effect of growing location and variety on nutritional and functional properties of proso millet (Panicum miliaceum) grown as a double crop. Cereal Chem. 2018, 95, 288–301. [Google Scholar] [CrossRef]
- Das, S.; Khound, R.; Santra, M.; Santra, D.K. Beyond Bird Feed: Proso Millet for Human Health and Environment. Agriculture 2019, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Almaski, A.; Thondre, S.; Lightowler, H.; Coe, S. Determination of the polyphenol and antioxidant activity of different types and forms of millet. Proc. Nutr. Soc. 2017, 76, E5. [Google Scholar] [CrossRef] [Green Version]
- Ramadoss, D.P.; Sivalingam, N. Vanillin extracted from Proso and Barnyard millets induce apoptotic cell death in HT-29 human colon cancer cell line. Nutr. Cancer 2019, 72, 1422–1437. [Google Scholar] [CrossRef]
- Anis, M.A.; Sreerama, Y.N. Inhibition of protein glycoxidation and advanced glycation end-product formation by barnyard millet (Echinochloa frumentacea) phenolics. Food Chem. 2020, 315, 126265. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Mohapatra, D.; Jadam, R.S.; Pandey, S.; Singh, V.; Kumar, V.; Kumar, A. Nutritional Composition of Millets. In Millets and Millet Technology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 101–119. [Google Scholar]
- Abah, C.R.; Ishiwu, C.N.; Obiegbuna, J.E.; Oladejo, A.A. Nutritional Composition, Functional Properties and Food Applications of Millet Grains. Asian Food Sci. J. 2020, 14, 9–19. [Google Scholar] [CrossRef]
- Saleh, A.S.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Amadou, I.; Gounga, M.E.; Le, G.-W. Millets: Nutritional Composition, Some Health Benefits and Processing—A Review. Emir. J. Food Agric. 2013, 25, 501–508. [Google Scholar] [CrossRef]
- Molla, M.M. Effect of Foxtail Millet Diet on Liver Injury and Blood Lipid Profile Induced by D-Galactosamine in Mice. Ph.D. Thesis, China Agricultural University (CAU), Beijing, China, 2016. [Google Scholar]
- Ciulu, M.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. Extraction and Analysis of Phenolic Compounds in Rice: A Review. Molecules 2018, 23, 2890. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2018, 275, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Slama, A.; Cherif, A.; Sakouhi, F.; Boukhchina, S.; Radhouane, L. Fatty acids, phytochemical composition and antioxidant potential of pearl millet oil. J. Consum. Prot. Food Saf. 2019, 15, 145–151. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, J.; Zheng, B.; Sun, J.; Luo, D.; Li, P.; Fan, J. Diversity of phenolics including hydroxycinnamic acid amide derivatives, phenolic acids contribute to antioxidant properties of proso millet. Lwt 2021, 154, 112611. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, M.; Apea-Bah, F.B.; Beta, T. Hydroxycinnamic acid amide (HCAA) derivatives, flavonoid C-glycosides, phenolic acids and antioxidant properties of foxtail millet. Food Chem. 2019, 295, 214–223. [Google Scholar] [CrossRef]
- Saha, D.; Gowda, M.V.C.; Arya, L.; Verma, M.; Bansal, K.C. Genetic and Genomic Resources of Small Millets. Crit. Rev. Plant Sci. 2016, 35, 56–79. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Naczk, M.; Shahidi, F. Effect of processing on the antioxidant activity of millet grains. Food Chem. 2011, 133, 1–9. [Google Scholar] [CrossRef]
- Abedin, J.; Abdullah, A.T.M.; Satter, M.A.; Farzana, T. Physical, functional, nutritional and antioxidant properties of foxtail millet in Bangladesh. Heliyon 2022, 8, e11186. [Google Scholar] [CrossRef]
- Liang, S.; Liang, K. Millet grain as a candidate antioxidant food resource: A review. Int. J. Food Prop. 2019, 22, 1652–1661. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Gujral, H.S. Influence of nutritional and antinutritional components on dough rheology and in vitro protein & starch digestibility of minor millets. Food Chem. 2019, 299, 125115. [Google Scholar] [CrossRef]
- Anitha, S.; Kane-Potaka, J.; Tsusaka, T.W.; Botha, R.; Rajendran, A.; Givens, D.I.; Parasannanavar, D.J.; Subramaniam, K.; Prasad, K.D.V.; Vetriventhan, M.; et al. A Systematic Review and Meta-Analysis of the Potential of Millets for Managing and Reducing the Risk of Developing Diabetes Mellitus. Front. Nutr. 2021, 8, 386. [Google Scholar] [CrossRef]
- Ren, X.; Yin, R.; Hou, D.; Xue, Y.; Zhang, M.; Diao, X.; Zhang, Y.; Wu, J.; Hu, J.; Hu, X.; et al. The Glucose-Lowering Effect of Foxtail Millet in Subjects with Impaired Glucose Tolerance: A Self-Controlled Clinical Trial. Nutrients 2018, 10, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shobana, S.; Harsha, M.R.; Platel, K.; Srinivasan, K.; Malleshi, N.G. Amelioration of Hyperglycaemia and Its Associated Complications by Finger Millet (Eleusine Coracana L.) Seed Coat Matter in Streptozotocin-Induced Diabetic Rats. Br. J. Nutr. 2010, 104, 1787–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Yin, R.; Liu, Z.; Niu, Y.; Guo, E.; Cheng, R.; Diao, X.; Xue, Y.; Shen, Q. Hypoglycemic Effect of Prolamin from Cooked Foxtail Millet (Setaria italic) on Streptozotocin-Induced Diabetic Mice. Nutrients 2020, 12, 3452. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.-H.; Ra, J.-E.; Lee, S.-J.; Lee, J.H.; Kim, S.R.; Lee, J.H.; Seo, W.D. Anti-hyperglycemic activity of polyphenols isolated from barnyard millet (Echinochloa utilis L.) and their role inhibiting α-glucosidase. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 571–579. [Google Scholar] [CrossRef]
- Krishnan, V.; Verma, P.; Saha, S.; Singh, B.; Vinutha, T.; Kumar, R.R.; Kulshreshta, A.; Singh, S.P.; Sathyavathi, T.; Sachdev, A.; et al. Polyphenol-Enriched Extract from Pearl Millet (Pennisetum Glaucum) Inhibits Key Enzymes Involved in Post Prandial Hyper Glycemia (α-Amylase, α-Glucosidase) and Regulates Hepatic Glucose Uptake. Biocatal. Agric. Biotechnol. 2022, 43, 102411. [Google Scholar] [CrossRef]
- Alzahrani, N.S.; Alshammari, G.M.; El-Ansary, A.; Yagoub, A.E.A.; Amina, M.; Saleh, A.; Yahya, M.A. Anti-Hyperlipidemia, Hypoglycemic, and Hepatoprotective Impacts of Pearl Millet (Pennisetum Glaucum L.) Grains and Their Ethanol Extract on Rats Fed a High-Fat Diet. Nutrients 2022, 14, 1791. [Google Scholar] [CrossRef]
- Park, K.-O.; Ito, Y.; Nagasawa, T.; Choi, M.-R.; Nishizawa, N. Effects of Dietary Korean Proso-Millet Protein on Plasma Adiponectin, HDL Cholesterol, Insulin Levels, and Gene Expression in Obese Type 2 Diabetic Mice. Biosci. Biotechnol. Biochem. 2008, 72, 2918–2925. [Google Scholar] [CrossRef]
- Anitha, S.; Botha, R.; Kane-Potaka, J.; Givens, D.I.; Rajendran, A.; Tsusaka, T.W.; Bhandari, R.K. Can Millet Consumption Help Manage Hyperlipidemia and Obesity? A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 478. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, I.-M.; Cha, Y.-S.; Park, Y. Millet consumption decreased serum concentration of triglyceride and C-reactive protein but not oxidative status in hyperlipidemic rats. Nutr. Res. 2010, 30, 290–296. [Google Scholar] [CrossRef]
- Nishizawa, N.; Togawa, T.; Park, K.-O.; Sato, D.; Miyakoshi, Y.; Inagaki, K.; Ohmori, N.; Ito, Y.; Nagasawa, T. Dietary Japanese Millet Protein Ameliorates Plasma Levels of Adiponectin, Glucose, and Lipids in Type 2 Diabetic Mice. Biosci. Biotechnol. Biochem. 2009, 73, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-Y.; Osada, K.; Ito, Y.; Nagasawa, T.; Choi, M.-R.; Nishizawa, N. Effects of Dietary Protein of Korean Foxtail Millet on Plasma Adiponectin, HDL-Cholesterol, and Insulin Levels in Genetically Type 2 Diabetic Mice. Biosci. Biotechnol. Biochem. 2005, 69, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.; Silva, M.M. An Overview on Applications and Side Effects of Antioxidant Food Additives. Emir. J. Food Agric. 2016, 28, 823. [Google Scholar] [CrossRef]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Nishizawa, N.; Oikawa, M.; Hareyama, S. Effect of Dietary Protein from Proso Millet on the Plasma Cholesterol Metabolism in Rats. Agric. Biol. Chem. 1990, 54, 229–230. [Google Scholar]
- Hou, D.; Chen, J.; Ren, X.; Wang, C.; Diao, X.; Hu, X.; Zhang, Y.; Shen, Q. A whole foxtail millet diet reduces blood pressure in subjects with mild hypertension. J. Cereal Sci. 2018, 84, 13–19. [Google Scholar] [CrossRef]
- Damsgaard, C.T.; Biltoft-Jensen, A.; Tetens, I.; Michaelsen, K.F.; Lind, M.V.; Astrup, A.; Landberg, R. Whole-Grain Intake, Reflected by Dietary Records and Biomarkers, Is Inversely Associated with Circulating Insulin and Other Cardiometabolic Markers in 8-to 11-Year-Old Children. J. Nutr. 2017, 147, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Duan, W.; Ren, X.; Wang, C.; Pan, Z.; Diao, X.; Shen, Q. Effect of foxtail millet protein hydrolysates on lowering blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2016, 56, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Khan, J.; Xue, Y.; Shen, Q. Consumption of mung bean (Vigna radiata L.) attenuates obesity, ameliorates lipid metabolic disorders and modifies the gut microbiota composition in mice fed a high-fat diet. J. Funct. Foods 2019, 64, 103687. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain ω-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Liu, B.; Ding, C.; Tang, W.; Zhang, C.; Gu, Y.; Wang, Z.; Yu, T.; Li, Z. Hepatic ROS Mediated Macrophage Activation Is Responsible for Irinotecan Induced Liver Injury. Cells 2022, 11, 3791. [Google Scholar] [CrossRef]
- Molla, M.M.; Ren, X.; Rahman, E.; Kamal, M.; Sabuz, A.A.; Khatun, A.; Chao, W.; Shen, Q. Use of Chou’s 5-steps Rule to Study the Effect of Cereal Dietary Protein on Liver and Coronary Heart Disease Prevention. Curr. Nutr. Food Sci. 2020, 17, 11–27. [Google Scholar] [CrossRef]
- Tuan, N.T.; Adair, L.S.; Stevens, J.; Popkin, B.M. Prediction of hypertension by different anthropometric indices in adults: The change in estimate approach. Public Health Nutr. 2009, 13, 639–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.; Koh, G. Clinical Evidence and Mechanisms of High-Protein Diet-Induced Weight Loss. J. Obes. Metab. Syndr. 2020, 29, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, B.; Li, L.; Mitchell, S.E.; Green, C.L.; D’Agostino, G.; Wang, G.; Wang, L.; Li, M.; Li, J.; et al. Very-Low-Protein Diets Lead to Reduced Food Intake and Weight Loss, Linked to Inhibition of Hypothalamic MTOR Signaling, in Mice. Cell Metab. 2021, 33, 888–904. [Google Scholar] [CrossRef]
- Chauhan, M.; Sonawane, S.K.; Arya, S.S. Nutritional and Nutraceutical Properties of Millets: A Review. Clin. J. Nutr. Diet. 2018, 1, 1–10. [Google Scholar]
- Shi, D.; Lv, L.; Fang, D.; Wu, W.; Hu, C.; Xu, L.; Chen, Y.; Guo, J.; Hu, X.; Li, A.; et al. Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 prevents CCl4-induced liver cirrhosis by protecting the intestinal barrier in rats. Sci. Rep. 2017, 7, 6927. [Google Scholar] [CrossRef] [Green Version]
- Massier, L.; Blüher, M.; Kovacs, P.; Chakaroun, R.M. Impaired Intestinal Barrier and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front. Endocrinol. 2021, 12, 616506. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.F.; Cotter, P.D.; Hogan, A.; O’Sullivan, O.; Joyce, A.; Fouhy, F.; Clarke, S.F.; Marques, T.M.; O’Toole, P.W.; Stanton, C.; et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut 2013, 62, 220–226. [Google Scholar] [CrossRef]
- Costa, M.C.; Arroyo, L.G.; Allen-Vercoe, E.; Stämpfli, H.R.; Kim, P.T.; Sturgeon, A.; Weese, J.S. Comparison of the Fecal Microbiota of Healthy Horses and Horses with Colitis by High Throughput Sequencing of the V3-V5 Region of the 16S rRNA Gene. PLoS ONE 2012, 7, e41484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middelbos, I.S.; Vester Boler, B.M.; Qu, A.; White, B.A.; Swanson, K.S.; Fahey Jr, G.C. Phylogenetic Characterization of Fecal Microbial Communities of Dogs Fed Diets with or without Supplemental Dietary Fiber Using 454 Pyrosequencing. PLoS ONE 2010, 5, e9768. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-M.; Shen, J.-D.; Xu, L.-P.; Li, H.-B.; Li, Y.-C.; Yi, L.-T. Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.F.; Walker, A.W.; Bosscher, D.; Garcia-Campayo, V.; Wagner, J.; Parkhill, J.; Duncan, S.H.; Flint, H.J. Relative Abundance of the Prevotella Genus within the Human Gut Microbiota of Elderly Volunteers Determines the Inter-Individual Responses to Dietary Supplementation with Wheat Bran Arabinoxylan-Oligosaccharides. BMC Microbiol. 2020, 20, 283. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, L.; Li, C.; Wu, H.; Ran, D.; Zhang, Z. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. AMB Express 2020, 10, 119. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]


| Millet Type | Features | References |
|---|---|---|
| Foxtail millet | Reduces risk of colon cancer. Lessen cholesterol and possesses anti-diabetic capability. Attenuates ethanol-induced hepatic damage. | [8,9] |
| Pearl millet | Gluten-free property averts celiac disease. The immune system improves by inhibiting pathogenicity induced by Shigella. | [10,11] |
| Finger millet | Reduces damage to soft tissue and facilitates the healing process. Reduces plasma triglycerides, thus reducing the risk of cardiovascular disease. | [12] |
| Kodo millet | Minimize glycemic index and diabetes occurrence, and have antioxidant actions as well. | [13] |
| Proso millet | Celiac disease can be prevented due to gluten-free properties. Being a low-glycemic index (GI) food reduces type 2 diabetes risks. | [14,15] |
| Little millet | Polyphenol content helps to prevent various metabolic disorders. | [13,16] |
| Barnyard millet | Damaging apoptotic cells reduces colorectal cancer risk. Inhibits protein glycation and glycoxidation, which improves the state of diabetes. | [17,18] |
| Food Grain | Arg | His | Lys | Trp | Phe | Tyr | Met | Cys | Thr | Leu | Ile | Val |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice | 480 | 130 | 230 | 80 | 280 | 290 | 150 | 90 | 230 | 500 | 300 | 280 |
| Wheat | 290 | 130 | 170 | 70 | 280 | 180 | 90 | 140 | 180 | 410 | 220 | 280 |
| Bajra | 300 | 140 | 190 | 110 | 290 | 200 | 150 | 110 | 140 | 750 | 260 | 330 |
| Sorghum | 240 | 160 | 150 | 70 | 300 | 180 | 100 | 90 | 210 | 880 | 270 | 340 |
| Finger millet | 300 | 130 | 220 | 100 | 310 | 220 | 210 | 140 | 240 | 690 | 400 | 480 |
| Kodo millet | 270 | 130 | 150 | 50 | 430 | - | 180 | 110 | 197 | 648 | 360 | 410 |
| Proso millet | 290 | 110 | 190 | 50 | 310 | - | 160 | - | 150 | 760 | 410 | 410 |
| Foxtail millet | 220 | 130 | 140 | 60 | 420 | - | 180 | 100 | 190 | 1040 | 480 | 430 |
| Little millet | 250 | 120 | 110 | 60 | 330 | - | 180 | 90 | 190 | 760 | 370 | 350 |
| Barnyard millet | 270 | 120 | 150 | 50 | 430 | - | 180 | 110 | 200 | 650 | 360 | 410 |
| Food Grain | Phenolic Acid (mg/100 g) | Reducing Capacity (%) | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vanillic | Proto Catechuic | P-Hydroxy Benzoic | Syringic | Gentisic | Gallic | Coumaric | Caffeic | Ferulic | Sinapinic | Cinnamic | |||
| Rice | 0.54 | 0.90 | 0.49 | 0.55 | NA | 1.38 | 1.62 | 0.61 | 11.48 | 2.08 | 0.30 | - | [24] |
| Finger millet | 1.52 | 2.31 | 0.89 | 0.77 | 6.15 | NA | 5.69 | 1.66 | 67.97 | NA | 3.50 | 5.7 ± 1.05 | [25] |
| Pearl millet | 1.63 | 1.18 | 2.20 | 1.73 | 9.63 | NA | 26.82 | 2.13 | 38.70 | NA | 34.53 | - | [26] |
| Proso millet | NA | NA | NA | 3.05 | NA | NA | 8.35 | 7.55 | 23.56 | NA | NA | 2.6 ± 0.20 | [27] |
| Foxtail millet | 8.71 | NA | 1.46 | 9.36 | 2.15 | NA | 213.37 | 1.06 | 75.58 | NA | 78.17 | 4.8 ± 1.15 | [28] |
| Food Grain | CHO (g) | Protein (g) | Fat (g) | Crude Fiber (g) | Dietary Fiber (g) | Energy (Kcal) | Mineral (g) | Calcium (mg) | Potassium (mg) | Iron (mg) | Phosphorus (mg) | Magnesium (mg) | Sodium (mg) | Zinc (mg) | Thiamin (mg) | Niacin (mg) | Riboflavin (mg) | Carotene (mg) | VB6 (mg) | Folic Acid | VE (mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice | 78.2 | 6.8 | 0.5 | 0.2 | 5.2 | 345 | 0.6 | 10 | 160 | 0.7 | 160 | 90 | - | 1.4 | 0.41 | 4.3 | 0.04 | 0 | - | 8.0 | - |
| Wheat | 71.2 | 11.8 | 1.5 | 1.2 | 12.9 | 346 | 1.5 | 41 | 306 | 5.3 | 306 | 138 | 17.1 | 2.7 | 0.41 | 5.1 | 0.1 | 64 | 0.57 | 36.6 | - |
| Bajra | 67.5 | 11.6 | 5.0 | 1.2 | - | 361 | 2.3 | 42 | 296 | 8.0 | 307 | 137 | 10.9 | 3.1 | 0.38 | 2.8 | 0.21 | 132 | - | 45.5 | 19.0 |
| Sorghum | 72.6 | 10.4 | 1.3 | 1.6 | 14.3 | 349 | 1.6 | 25 | 222 | 4.1 | 266 | 171 | 7.3 | 1.6 | 0.38 | 4.3 | 0.15 | 47 | 0.21 | 20.0 | 12.0 |
| Finger millet | 72.0 | 7.3 | 1.3 | 3.6 | 18.8 | 328 | 2.7 | 344 | 283 | 3.9 | 283 | 137 | 11.0 | 2.3 | 0.42 | 1.1 | 0.19 | 42 | - | 18.3 | 22.0 |
| Kodo millet | 65.9 | 8.3 | 1.4 | 9.0 | 15 | 309 | 2.6 | 27 | 188 | 0.5 | 188 | 147 | 4.6 | 0.7 | 0.15 | 2.0 | 0.09 | 0 | - | 23.1 | - |
| Proso millet | 70.4 | 12.5 | 1.1 | 2.2 | 14.2 | 341 | 1.9 | 14 | 206 | 0.8 | 206 | 153 | 8.2 | 1.4 | 0.41 | 4.5 | 0.28 | 0 | - | - | - |
| Foxtail millet | 60.9 | 12.3 | 4.3 | 8.0 | 14 | 331 | 3.3 | 31 | 290 | 2.8 | 290 | 81 | 4.6 | 2.4 | 0.59 | 3.2 | 0.11 | 32 | - | 15.0 | 31.0 |
| Little millet | 67.0 | 7.7 | 4.7 | 7.6 | 12.2 | 341 | 1.5 | 17 | 220 | 9.3 | 220 | 133 | 8.1 | 3.7 | 0.30 | 3.2 | 0.09 | 0 | - | 9.0 | - |
| Barnyard millet | 65.5 | 6.2 | 2.2 | 9.8 | 13.7 | 307 | 4.4 | 20 | 280 | 5.0 | 280 | 82 | - | 3.0 | 0.33 | 4.2 | 0.1 | 0 | - | - | - |
| Diet Group | Fasting Blood Glucose (mmol/L) | Insulin (mu/L) | Islet β Cell Function (HOMA-β) | Blood Glucose 0, 30, 60 and 120 min (mmol/L) | Area under Curve (AUC) | Serum Triglyceride (mmol/L) | Liver Triglyceride (TG) (mmol/L) | Aspartate Amino-Transferase (U/L) | Alanine Amino-Transferase (U/L) |
|---|---|---|---|---|---|---|---|---|---|
| NC | 7.20 | 16.20 | 135.45 | 5, 10, 8, 7 | 18.00 | 0.70 | 1.20 | 56.00 | 30.00 |
| MC | 27.30 | 16.35 | 15.24 | 27, 32, 31, 30 | 60.00 | 1.60 | 3.00 | 80.00 | 77.00 |
| PCFM | 23.40 | 18.25 | 20.23 | 21, 29, 25, 23 | 56.00 | 1.00 | 2.00 | 54.00 | 56.00 |
| Parameter | Dietary Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0T0 | GNT0 | GNT1 | G1T1 | G2T2 | G3T3 | G4T4 | G5T5 | G6T6 | ||
| Activities of serum enzyme (U/L) | AST | 340.83 ± 34.94 d | 113.33 ± 25.21 ab | 261.25 ± 21.13 c | 241.10 ± 7.30 b | 243.16 ± 211.32 b | 81.33 ± 5.24 a | 94.66 ± 3.14 a | 67.66 ± 14.27 a | 85.50 ± 11.39 a |
| ALT | 55.0 ± 18.56 d | 48.33 ± 23.44 cd | 50.33 ± 10.59 c | 50.33 ± 21.36 c | 51.66 ± 3.14 c | 18.16 ± 7.38 ab | 22.16 ± 2.40 b | 13.0 ± 4.0 a | 21.66 ± 1.50 b | |
| LDH | 2832.16 ± 347.53 d | 1531.83 ± 307.60 bc | 2585.16 ± 1020.30 c | 2232.5 ± 461.59 cd | 2298.66 ± 259.77 cd | 1114.33 ± 314.91 ab | 1283.83 ± 87.44 b | 1047.0 ± 95.33 a | 1107.66 ± 193.95 ab | |
| Plasma components (mmol/L) | TC | 4.34 ± 0.19 d | 3.45 ± 021 bc | 4.12 ± 1.39 c | 3.98 ± 0.42 bc | 3.47 ± 0.28 bc | 2.62 ± 0.16 ab | 3.06 ± 0.07 b | 2.05 ± 0.32 a | 2.85 ± 0.10 ab |
| TG | 1.25 ± 0.20 d | 1.13 ± 0.23 cd | 1.20 ± 0.15 c | 0.84 ± 0.14 b | 0.85 ± 0.08 b | 0.82 ± 0.08 ab | 0.83 ± 0.15 b | 0.81 ± 0.16 a | 0.83 ± 0.07 b | |
| HDL-C | 0.72 ± 0.54 | 1.34 ± 1.19 | 0.75 ± 0.35 | 1.55 ± 1.63 | 1.67 ± 1.80 | 2.11 ± 1.58 | 2.07 ± 1.46 | 3.17 ± 1.48 | 2.96 ± 1.83 | |
| LDL-C | 2.81 ± 1.28 d | 1.81 ± 1.28 bc | 2.33 ± 1.13 c | 1.94 ± 1.03 b | 2.01 ± 1.03 bcd | 0.48 ± 0.39 a | 0.68 ± 0.60 a | 0.47 ± 0.22 a | 0.68 ± 0.23 a | |
| Liver lipids (mmol/L) | TC | 0.92 ± 0.56 | 0.44 ± 0.14 | 0.51 ± 0.36 | 0.42 ± 0.15 | 0.38 ± 0.23 | 0.36 ± 0.14 | 0.36 ± 0.22 | 0.16 ± 0.15 | 0.26 ± 0.16 |
| TG | 1.50 ± 0.98 | 1.08 ± 0.60 | 1.67 ± 1.15 | 0.92 ± 0.56 | 1.27 ± 1.48 | 0.74 ± 0.34 | 0.85 ± 0.30 | 0.38 ± 0.26 | 0.76 ± 0.55 | |
| Liver MDA (nmol/mg protein) | 5.21 ± 0.65 d | 4.35 ± 1.46 cd | 4.56 ± 0.67 c | 4.68 ± 1.13 c | 4.58 ± 1.37 c | 2.55 ± 1.65 b | 2.89 ± 0.32 b | 0.68 ± 0.21 a | 2.79 ± 0.08 b | |
| Parameter | Dietary Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0T0 | GNT0 | GNT1 | G1T1 | G2T2 | G3T3 | G4T4 | G5T5 | G6T6 | ||
| Activities of serum enzyme (U/L) | AST | 133.16 ± 56.33 | 126.00 ± 23.09 | 128.66 ± 34.56 | 125.66 ± 38.68 | 111.83 ± 36.21 | 121.16 ± 22.56 | 120.33 ± 24.32 | 106.33 ± 27.50 | 102.66 ± 21.78 |
| ALT | 62.16 ± 12.44 | 43.00 ± 52.50 | 48.50 ± 17.62 | 40.83 ± 11.97 | 42.83 ± 40.57 | 34.50 ± 12.58 | 29.16 ± 15.31 | 25.50 ± 12.37 | 32.66 ± 10.30 | |
| LDH | 1338.66 ± 323.80 | 1210.16 ± 286.14 | 1334.50 ± 328.57 | 1104.66 ± 277.13 | 1117.66 ± 226.02 | 904.33 ± 425.28 | 960.83 ± 151.03 | 903.16 ± 95.10 | 1019.50 ± 244.42 | |
| Plasma components (mmol/L) | TC | 3.79 ± 0.62 d | 3.11 ± 0.33 bc | 3.44 ± 0.53 c | 3.03 ± 0.50 bc | 3.04 ± 1.04 bc | 2.46 ± 0.69 ab | 2.68 ± 0.36 b | 2.11 ± 0.17 a | 2.61 ± 0.92 b |
| TG | 1.50 ± 0.74 | 1.27 ± 0.80 | 1.28 ± 0.45 | 1.21 ± 0.42 | 1.20 ± 0.12 | 0.95 ± 0.22 | 1.00 ± 0.14 | 0.87 ± 0.22 | 0.93 ± 0.27 | |
| HDL-C | 2.75 ± 0.64 a | 3.10 ± 0.45 ab | 3.05 ± 1.01 ab | 3.17 ± 0.45 ab | 3.12 ± 2.48 ab | 4.11 ± 0.81 b | 4.08 ± 1.05 b | 8.06 ± 6.97 c | 7.65 ± 2.34 bc | |
| LDL-C | 10.63 ± 7.61 c | 10.41 ± 4.05c | 10.51 ± 3.26 c | 5.59 ± 1.85 b | 4.90 ± 3.05 b | 0.20 ± 0.04 a | 0.26 ± 0.09 a | 0.10 ± 0.0 a | 0.14 ± 0.05 a | |
| Liver lipids(mmol/L) | TC | 0.91 ± 0.28 | 0.77 ± 0.15 | 0.83 ± 0.16 | 0.81 ± 0.20 | 0.76 ± 0.21 | 0.62 ± 0.06 | 0.72 ± 0.16 | 0.54 ± 0.28 | 0.72 ± 0.16 |
| TG | 6.74 ± 0.50 d | 3.51 ± 1.03 b | 4.61 ± 2.66 bc | 3.74 ± 2.26 b | 5.26 ± 6.04 c | 1.30 ± 1.03 ab | 1.68 ± 0.61 ab | 0.52 ± 0.60 a | 0.96 ± 0.80 ab | |
| Liver MDA (nmol/mg protein) | 5.31 ± 1.22 d | 3.03 ± 1.16 cd | 3.13 ± 1.58 cd | 3.43 ± 1.46 c | 3.47 ± 1.43 c | 1.38 ± 0.87 ab | 1.81 ± 0.77 b | 0.58 ± 0.14 a | 1.27 ± 0.84 ab | |
| Diet | Bacteroidetes % | Firmicutes % | Verrucomicrobia % | Proteobacteria % | Actinobacteria % | Cyanobacteria % | Tenericutes % | Other % |
|---|---|---|---|---|---|---|---|---|
| Normal | 54.82 | 31.23 | 9.24 | 2.9 | 1.05 | 0.11 | 0 | 0.65 |
| Casein | 85.94 | 12.61 | 0.01 | 0.39 | 0.09 | 0.01 | 0.01 | 0.94 |
| Control | 57.89 | 16.80 | 24.44 | 0.30 | 0.33 | 0.01 | 0.03 | 0.20 |
| UC FM flour | 46.10 | 49.88 | - | 0.87 | 0.84 | 0.01 | - | 2.30 |
| C FM flour | 80.81 | 5.63 | - | 3.65 | 8.30 | - | - | 1.61 |
| UCE FM protein | 68.07 | 12.87 | 0.04 | 18.18 | 0.67 | 0.01 | - | 0.16 |
| CE FM protein | 79.35 | 14.26 | - | 5.62 | 0.14 | 0.01 | - | 0.62 |
| CEE FM protein | 69.46 | 22.48 | 4.53 | 1.79 | 0.58 | - | - | 1.16 |
| UCEE FM protein | 0.96 | 34.55 | 63.40 | 0.50 | 0.57 | - | - | 0.02 |
| Diet | Akkermansia (%) | Allobaculum (%) | Prevotella (%) | Bacteroides (%) | Lactobacillus (%) | Oscilliospria (%) | Anaerotruncus (%) | Sutterella (%) |
|---|---|---|---|---|---|---|---|---|
| Normal | 9.24 | 24.14 | 0.85 | 2.99 | 1.84% | 0.34 | 0.29 | - |
| Casein | 0.01 | 0.01 | 10.96 | 5.53 | 0.19 | 0.65 | 0.01 | - |
| Control | 24.45 | - | 1.68 | 1.21 | 10.93 | 0.38 | 0.04 | 0.12 |
| UC FM flour | - | 38.39 | 0.84 | 0.13 | 4.96 | 0.17 | 0.04 | 0.26 |
| C FM flour | - | 0.91 | 12.05 | 0.81 | 0.70 | 0.14 | 0.01 | 3.52 |
| UCE FM protein | 0.04 | - | 6.33 | 4.82 | 0.01 | 4.84 | 2.13 | 14.02 |
| CE FM protein | - | - | 0.51 | 29.87 | - | 1.94 | 0.43 | 0.18 |
| CEEFM protein | 4.53 | - | 54.92 | 3.64 | - | 0.54 | 1.12 | 1.56 |
| UCEE FM protein | 63.40 | - | - | 0.25 | 0.01 | 1.50 | 0.38 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabuz, A.A.; Rana, M.R.; Ahmed, T.; Molla, M.M.; Islam, N.; Khan, H.H.; Chowdhury, G.F.; Zhao, Q.; Shen, Q. Health-Promoting Potential of Millet: A Review. Separations 2023, 10, 80. https://doi.org/10.3390/separations10020080
Sabuz AA, Rana MR, Ahmed T, Molla MM, Islam N, Khan HH, Chowdhury GF, Zhao Q, Shen Q. Health-Promoting Potential of Millet: A Review. Separations. 2023; 10(2):80. https://doi.org/10.3390/separations10020080
Chicago/Turabian StyleSabuz, Ashfak Ahmed, Md Rahmatuzzaman Rana, Tanvir Ahmed, Mohammad Mainuddin Molla, Nazmul Islam, Hafizul Haque Khan, Golam Ferdous Chowdhury, Qingyu Zhao, and Qun Shen. 2023. "Health-Promoting Potential of Millet: A Review" Separations 10, no. 2: 80. https://doi.org/10.3390/separations10020080
APA StyleSabuz, A. A., Rana, M. R., Ahmed, T., Molla, M. M., Islam, N., Khan, H. H., Chowdhury, G. F., Zhao, Q., & Shen, Q. (2023). Health-Promoting Potential of Millet: A Review. Separations, 10(2), 80. https://doi.org/10.3390/separations10020080