Pharmacognostical and Phytochemical Evaluation of a Unani Polyherbal Formulation: Dawa ul Kurkum by HPTLC
Abstract
:1. Introduction
2. Materials and Methods
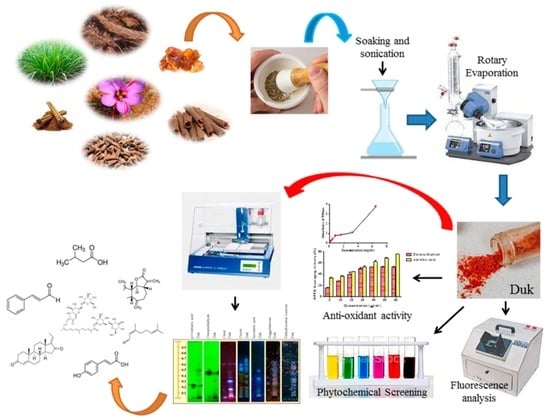
2.1. Collection of Plant Material, Drug Authentication, and Preparation of Duk
2.2. Organoleptic Properties
2.3. Fluorescence Analysis
2.4. Preliminary Phytochemical Screening
2.5. Quantitative Phytochemical Analysis
2.5.1. Total Phenol Content (TPC)
2.5.2. Total Flavonoid Content (TFC)
2.6. Evaluation of Antioxidants
2.6.1. DPPH Radical Scavenging Assay
2.6.2. Ferrous Reducing Antioxidant Capacity (FRAC) Assay
2.7. Phytochemical Profiling of DuK through High-Performance Thin-Layer Chromatography (HPTLC)
3. Results & Discussion
3.1. Organoleptic Evaluation
3.2. Fluorescence Analysis
3.3. Preliminary and Quantitative Phytochemical Screening and Antioxidant Activity of Duk
3.4. HPTLC of Duk Showed the Presence of All Seven Principal Active Metabolites of Its Constituent Herbs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, S.; Chakraborty, R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. J. Tradit. Complement. Med. 2017, 7, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Shetty, H. Use of natural products for oral hygiene maintenance: Revisiting traditional medicine. J. Tradit. Complement. Med. 2018, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The World Medicines Situation (No. WHO/EDM/PAR/2004.5). Available online: https://www.who.int/publications-detail-redirect/WHO-EDM-PAR-2004.5 (accessed on 15 March 2022).
- Tabish, S.A. Complementary and alternative healthcare: Is it evidence-based? Int. J. Health Sci. 2008, 2, 5–9. [Google Scholar]
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023. Available online: https://www.who.int/publications/i/item/9789241506096 (accessed on 15 March 2022).
- Keene, M.R.; Heslop, I.M.; Sabesan, S.S.; Glass, B.D. Complementary and alternative medicine use in cancer: A systematic review. Complement. Ther. Clin. Pract. 2019, 35, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Sheth, P.A.; Pawar, A.T.; Mote, C.S.; More, C. Antianemic activity of polyherbal formulation, Raktavardhak Kadha, against phenylhydrazine-induced anemia in rats. J. Ayurveda Integr. Med. 2021, 12, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Nissen, L.; McCarthy, A.; Steadman, K.; Windsor, C. Exploring the use of complementary and alternative medicine in cancer patients. Integr. Cancer Ther. 2019, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Formulary of Unani Medicine-I. Central Council for Research in Unani Medicine (CCRUM); Ministry of Health and Family Welfare, Department of AYUSH: New Delhi, India, 2006; p. 88.
- Kabiruddin, M. Bayaz e Kabeer-II; Shaikh Mohammad Bashir and Sons: Lahore, Pakistan, 2018; p. 354. [Google Scholar]
- Reshi, M.R.; Gulati, K.; Naqvi, M.; Hassan, N.; Ray, A. Hepatoprotective Effects of Dawa-Ul-Kurkum, A Unani Polyherbal Preparation and the Possible Mechanisms in Experimental Model of Ethanol Induced Liver Damage in Rats. J. Pharm. Pharmacol. Res. 2022, 6, 122–130. [Google Scholar] [CrossRef]
- Reshi, M.R.; Gulati, K.; Ray, A. Immunomodulation During Hepatoprotective Effects of Dawa-Ul-Kurkum in D-Galactosamine-Induced Liver Cirrhosis in Rats. Int. J. Toxicol. Pharmacol. Res. 2022, 12, 166–179. [Google Scholar]
- Srivastava, R.; Ahmed, H.; Dixit, R.K.; Saraf, S.A. Crocus sativus L.: A comprehensive review. Pharmacogn. Rev. 2010, 4, 200. [Google Scholar] [CrossRef] [Green Version]
- Khorasany, A.R.; Hosseinzadeh, H. Therapeutic effects of saffron (Crocus sativus L.) in digestive disorders: A review. Iran J. Basic Med. Sci. 2016, 19, 455–469. [Google Scholar]
- Finley, J.W.; Gao, S. A perspective on Crocus sativus L. (Saffron) constituent crocin: A potent water-soluble antioxidant and potential therapy for Alzheimer’s disease. J. Agric. Food Chem. 2017, 65, 1005–1020. [Google Scholar] [CrossRef]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Jaafar, H.Z. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 2010, 15, 6244–6256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaddonfard, E.; Farshid, A.A.; Eghdami, K.; Samadi, F.; Erfanparast, A. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacol. Rep. 2013, 65, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Hosseinzadeh, H.; Movassaghi, A.R.; Imenshahidi, M.; Abnous, K. Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure. Chem.-Biol. Interact. 2013, 203, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Lekhak, M.M.; Chahal, S.; Goutam, U.; Jha, P.; Naidoo, D.; Ochatt, S.J.; Kumar, V. Nardostachys jatamansi (D. Don) DC.: An invaluable and constantly dwindling resource of the Himalayas. S. Afr. J. Bot. 2020, 135, 252–267. [Google Scholar] [CrossRef]
- Jugran, A.K.; Rawat, S.; Bhatt, I.D.; Rawal, R.S. Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phytother. Res. 2019, 33, 482–503. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, R.; Mishra, S. Pharmacological properties and their medicinal uses of Cinnamomum: A review. J. Pharm. Pharmacol. 2019, 71, 1735–1761. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Su, B.; Jiang, H.; Cui, N.; Yu, Z.; Yang, Y.; Sun, Y. Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): A review. Fitoterapia 2020, 146, 104675. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Sharma, H. A Review on Medicinal Uses of Cinnamomum verum (Cinnamon). J. Drug Deliv. Ther. 2021, 11, 161–166. [Google Scholar] [CrossRef]
- Avoseh, O.; Oyedeji, O.; Rungqu, P.; Nkeh-Chungag, B.; Oyedeji, A. Cymbopogon species; ethnopharmacology, phytochemistry and the pharmacological importance. Molecules 2015, 20, 7438–7453. [Google Scholar] [CrossRef]
- Kumar, S.; Shankar, V. Medicinal plants of the Indian desert: Commiphora wightii (Arnott) Bhand. J. Arid Environ. 1982, 5, 1–11. [Google Scholar] [CrossRef]
- Madhuri, K.; Elango, K.; Ponnusankar, S. Saussurea lappa (Kuth root): Review of its traditional uses, phytochemistry and pharmacology. Orient. Pharm. Exp. Med. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Aziz, N.; Wal, A.; Wal, P.; Pal, R.S. Preparation and evaluation of the polyherbal powder: The nature’s pharmacy for the treatment of diabetes mellitus and its complications. Pharmacophores 2019, 10, 60–70. [Google Scholar]
- Maurya, N.; Vishwakarma, R.; Bhanap, K.; Shah, S.; Patil, S. Preparation and quality evaluation of hingwashtak churna: A polyherbal formulation. J. Pharmacogn. Phytochem. 2020, 9, 1923–1927. [Google Scholar]
- Wallis, T.E. Text Book of Pharmacognosy, 5th ed.; CBS Publishers and Distributors: New Delhi, India, 2004. [Google Scholar]
- Sharma, B.; Bagchi, A.; Bhutia, S.; Sarkar, B.R.; Pal, P. Formulation and Evaluation of Expectorant activity of Poly Herbal Cough Syrup from Traditional Medicinal Plant extracts of North East India. Res. J. Pharm. Technol. 2022, 15, 949–953. [Google Scholar] [CrossRef]
- Chanthasri, W.; Puangkeaw, N.; Kunworarath, N.; Jaisamut, P.; Limsuwan, S.; Maneenoon, K.; Choochana, P.; Chusri, S. Antioxidant capacities and total phenolic contents of 20 polyherbal remedies used as tonics by folk healers in Phatthalung and Songkhla provinces, Thailand. BMC Complement. Altern. Med. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Rahim, N.F.; Muhammad, N.; Abdullah, N.; Talip, B.A.; Poh, K.H. The interaction effect and optimal formulation of selected polyherbal extracts towards antioxidant activity. Food Res. 2020, 4, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Arawwawala, L.D.; Karunaratne, T.D.; Sugatharatana, K.; Ariyawansa, H.S.; De Silva, H.A. In vitro antioxidant activities of a poly herbal preparation: Sarasvatha Choorna. Free Radic. Antioxid. 2021, 11, 35–47. [Google Scholar] [CrossRef]
- Kumar, H.D.; Mannem, K. A comparison study of macroscopical and microscopical characteristics of powder of Haritaki: Terminalia chebula (pericarp), Yavani: Ttrachyspermum ammi (fruit), Ajmoda: Apium leptophyllum (fruit) and Sunthi: Zingiber officinalie (rhizome). Int. J. Ayurveda Pharma. Res. 2012, 3, 309–313. [Google Scholar]
- Subba, A.; Mandal, P. Pharmacognostic studies and in vitro antioxidant potential of traditional polyherbal formulation of west Sikkim with Asparagus Spp. Pharmacogn. J. 2015, 7, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Shalini, K.; Ilango, K. Preliminary phytochemical studies, GC-MS analysis and in vitro antioxidant activity of selected medicinal plants and its polyherbal formulation. Pharmacogn. J. 2021, 13, 648–659. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutt, V.; Srivastav, S.; Mittal, S.; Haque, M.R. Determination of microbial load, total Phenolic and flavonoids contents in polyherbal formulation “yograj guggulu vati”. J. Drug Deliv. Ther. 2020, 10, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Gökbulut, A. High Performance Thin Layer Chromatography (HPTLC) for the investigation of medicinal plants. Curr. Anal. Chem. 2021, 17, 1252–1259. [Google Scholar] [CrossRef]
- Ahmad Dar, A.; Sangwan, P.L.; Kumar, A. Chromatography: An important tool for drug discovery. J. Sep. Sci. 2020, 43, 105–119. [Google Scholar] [CrossRef]
- Girme, A.; Ghule, C.; Gaikar, N.; Hingorani, L. Rapid and sensitive High-Performance Thin-Layer Chromatographic (HPTLC) method for identification and quantification of luteolin by densitometry in Kasamarda (Cassia occidentalis L.). Indian J. Tradit. Knowl. 2021, 20, 700–706. [Google Scholar]



| S. No. | Biological Name of the Drug | Common Name/ Unani Name | Part of the Plant | Weight (g)Used/ 50 g |
|---|---|---|---|---|
| 1. | Cinnamomum cassia (L.) J. Presl | Chinese cinnamon/ Taj Qalmi | Bark | 10 |
| 2. | Cinnamomum zeylanicum Blume | True cinnamon/Darchini | Bark | 5 |
| 3. | Cymbopogon jwarancusa (Jones ex Roxb.) Schult. | Lemongrass/Izkhar | Aerial part | 5 |
| 4. | Crocus sativa L. | Saffron/Zafran | Stigma | 10 |
| 5. | Commiphora wightii (Arn.) Bhandari | Guggul/Murmakki | Resin | 5 |
| 6. | Nardostachys jatamansi (D.Don) DC. | Jatamansi/ Sumbuluttib | Root | 10 |
| 7. | Saussurea lappa (Decne.) Sch.Bip. | Costus/Qust | Root | 5 |
| S. No. | Test for | Procedure | Observation for the Presence |
|---|---|---|---|
| 1. | Carbohydrates | 2 mL of Duk solution + 1 mL of Molisch’s reagent + Few drops of concentrated sulphuric acid | Purple or reddish colour |
| 2. | Tannins | 1 mL Duk solution + 2 mL of 5% ferric chloride | Dark blue or greenish colour |
| 3. | Saponins | 2 mL of Duk solution + 2 mL of distilled water; Shake for 15 min | 1 cm layer of foam |
| 4. | Flavonoids | 2 mL of Duk solution + 1 mL of 2N sodium hydroxide | Yellow colour |
| 5. | Alkaloids | 2 mL of Duk solution + 2 mL of concentrated hydrochloric acid + Few drops of Mayer’s reagent | Green colour or white precipitate |
| 6. | Quinones | 1 mL Duk solution + 1 mL of concentrated sulphuric acid | Red colour |
| 7. | Glycosides | 2 mL of Duk solution + 3 mL of chloroform + 10% ammonia solution | Pink colour |
| 8. | Cardiac glycosides | 0.5 mL of Duk solution + 2 mL of glacial acetic acid + Few drops of 5% ferric chloride + 1 mL of concentrated sulphuric acid | Brown ring at the interface |
| 9. | Terpenoids | 0.5 mL of Duk solution + 2 mL of chloroform + concentrated sulphuric acid | Red brown colour at the interface |
| 10. | Phenols | 1 mL Duk solution + 2 mL of distilled water + Few drops of 10% ferric chloride | Blue or green colour |
| 11. | Coumarins | 1 mL Duk solution + 1 mL of 10% NaOH | Yellow colour |
| 12. | Steroids and phytosterols | 1 mL Duk solution + 1 mL of chloroform + Few drops of concentrated sulphuric acid | Brown ring: Steroids Bluish brown ring: Phytosterols |
| 13. | Phlobatannins | 1 mL Duk solution + Few drops of 2% HCl | Red colour precipitate |
| 14. | Anthraquinones | 1 mL Duk solution + 10% ammonia solution | Pink colour precipitate |
| S. No. | Active Constituent | Solvent System | Ratio | Application Volume | Illumination | Rf Value | Post-Chromatographic Derivatization | |
|---|---|---|---|---|---|---|---|---|
| Standard | Duk | |||||||
| 1. | p-Coumaric acid | Toluene: Ethyl Acetate: Formic Acid | 8:2:0.5 | 25 μL | 15 μL | 254 nm | 0.28 | Anisaldehyde Sulfuric Reagent |
| 2. | Cinnamaldehyde | Toluene: Ethyl Acetate | 9.5:0.5 | 15 μL | 10 μL | 254 nm | 0.48 | Vanillin Sulfuric Reagent |
| 3. | Citral | Toluene: Ethyl Acetate | 9.7:0.3 | 15 μL | 15 μL | 366 nm | 0.62 | Vanillin Sulfuric Reagent |
| 4. | Crocin | n-Butanol: Water: Acetic acid | 4:1:1 | 15 μL | 10 μL | 366 nm | 0.28 | Anisaldehyde Sulfuric Reagent |
| 5. | Guggulsterone | Hexane: Ethyl Acetate: Acetic Acid | 75:25:0.5 | 10 μL | 10 μL | 366 nm | z: 0.39 e: 0.29 | Anisaldehyde Sulfuric Reagent |
| 6. | Isovaleric acid | Toluene: Acetone | 9:1 | 15 μL | 15 μL | 366 nm | 0.11 | Anisaldehyde Sulfuric Reagent |
| 7. | Dehydrocostus lactone | Toluene: Ethyl Acetate | 9:1 | 20 μL | 20 μL | 366 nm | 0.56 | Anisaldehyde Sulfuric Reagent |
| S. No. | Treatment Conditions | Daylight | UV Light | |
|---|---|---|---|---|
| 254 nm | 366 nm | |||
| 1. | Duk as such | Reddish orange | Rust Brown | Blackish-brown |
| 2. | Duk + 1N NaOH (aq.) | Brownish-yellow | Brown | Blackish-brown |
| 3. | Duk + 1N NaOH (alc.) | Reddish-brown | Reddish-brown | Blackish-brown |
| 4. | Duk + 1N HCl | Deep orange | Dark orange | Brown |
| 5. | Duk + NH3 | Light brown | Light brown | Brown |
| 6. | Duk + 5% Iodine | Deep brown | Brown | Brown |
| 7. | Duk + 5% FeCl3 | Blackish-brown | Brownish-black | Black |
| 8. | Duk + Acetic aid | Brownish-orange | Brownish-orange | Blackish- orange |
| 9. | Duk + 1N H2SO4 | Orange | Reddish-brown | Blackish-brown |
| 10. | Duk + 1N HNO3 | Yellowish-orange | Reddish-orange | Reddish-black |
| S. No. | Phytochemical Tests | Duk |
|---|---|---|
| 1. | Carbohydrates | +ve |
| 2. | Tannins | −ve |
| 3. | Saponins | −ve |
| 4. | Flavonoids | +ve |
| 5. | Alkaloids | −ve |
| 6. | Quinones | +ve |
| 7. | Glycosides | +ve |
| 8. | Cardiac glycosides | +ve |
| 9. | Terpenoids | +ve |
| 10. | Phenols | +ve |
| 11. | Coumarins | +ve |
| 12. | Steroids and phytosterols | Phytosterols |
| 13. | Phlobatannins | −ve |
| 14. | Anthraquinones | −ve |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, M.; Sumaiya, S.; Ali, S.; Naved, T.; Sharma, A.; Ahmad, A.; Sikander, M.; Sarwat, M. Pharmacognostical and Phytochemical Evaluation of a Unani Polyherbal Formulation: Dawa ul Kurkum by HPTLC. Separations 2023, 10, 89. https://doi.org/10.3390/separations10020089
Gupta M, Sumaiya S, Ali S, Naved T, Sharma A, Ahmad A, Sikander M, Sarwat M. Pharmacognostical and Phytochemical Evaluation of a Unani Polyherbal Formulation: Dawa ul Kurkum by HPTLC. Separations. 2023; 10(2):89. https://doi.org/10.3390/separations10020089
Chicago/Turabian StyleGupta, Meenakshi, Sajida Sumaiya, Sher Ali, Tanveer Naved, Archana Sharma, Ajaz Ahmad, Mohammed Sikander, and Maryam Sarwat. 2023. "Pharmacognostical and Phytochemical Evaluation of a Unani Polyherbal Formulation: Dawa ul Kurkum by HPTLC" Separations 10, no. 2: 89. https://doi.org/10.3390/separations10020089
APA StyleGupta, M., Sumaiya, S., Ali, S., Naved, T., Sharma, A., Ahmad, A., Sikander, M., & Sarwat, M. (2023). Pharmacognostical and Phytochemical Evaluation of a Unani Polyherbal Formulation: Dawa ul Kurkum by HPTLC. Separations, 10(2), 89. https://doi.org/10.3390/separations10020089