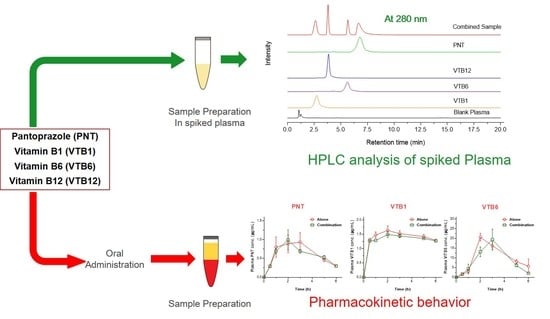
Simultaneous Pharmacokinetic Evaluation of Pantoprazole and Vitamin B Complex for Assessing Drug–Drug Interactions in Healthy Bangladeshi Adults by a Newly Developed and Validated HPLC Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Drug–Drug Interaction Study
2.2.1. Sample Preparation
2.2.2. X-ray Powder Diffraction (XRPD)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3. Method Development
2.3.1. Instrumentation
2.3.2. Preparation of Stock and Working Standard Solutions
2.3.3. Chromatographic Separation
2.4. Method Validation
2.5. Pharmacokinetic and Drug–Drug Interaction Studies
2.5.1. Study Design
2.5.2. Volunteers
2.5.3. Collection of Blood Sample
2.5.4. Ethical Declaration
2.5.5. Safety Parameters
2.5.6. Plasma Sample Preparation
2.5.7. Determination of Pharmacokinetic Parameters
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Interaction of the Solid Samples
3.1.1. Solid-State Characterization
3.1.2. Drug-Drug Interaction Using FT-IR
3.2. Method Development
Optimization of Separation Conditions
3.3. Method Validation
Precision, Accuracy, Recovery and Robustness
3.4. Pharmacokinetic and DDI Studies in Healthy Adults
3.5. Safety Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esumi, S.; Ushio, S.; Zamami, Y. Polypharmacy in Older Adults with Alzheimer’s Disease. Medicina 2022, 58, 1445. [Google Scholar] [CrossRef] [PubMed]
- Villén, N.; Roso-Llorach, A.; Gallego-Moll, C.; Danes-Castells, M.; Fernández-Bertolin, S.; Troncoso-Mariño, A.; Monteagudo, M.; Amado, E.; Violán, C. Polypharmacy Patterns in Multimorbid Older People with Cardiovascular Disease: Longitudinal Study. Geriatrics 2022, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Lleal, M.; Baré, M.; Ortonobes, S.; Sevilla-Sánchez, D.; Jordana, R.; Herranz, S.; Gorgas, M.Q.; Espaulella-Ferrer, M.; Arellano, M.; de Antonio, M.; et al. Comprehensive Multimorbidity Patterns in Older Patients Are Associated with Quality Indicators of Medication—MoPIM Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 15902. [Google Scholar] [CrossRef]
- Benny Gerard, N.; Mathers, A.; Laeer, C.; Lui, E.; Kontio, T.; Patel, P.; Dolovich, L. A Descriptive Quantitative Analysis on the Extent of Polypharmacy in Recipients of Ontario Primary Care Team Pharmacist-Led Medication Reviews. Pharmacy 2020, 8, 110. [Google Scholar] [CrossRef]
- Khandeparkar, A.; Rataboli, P. V A study of harmful drug-drug interactions due to polypharmacy in hospitalized patients in Goa Medical College. Perspect. Clin. Res. 2017, 8, 180–186. [Google Scholar] [CrossRef]
- Georgiev, K.D.; Hvarchanova, N.; Stoychev, E.; Kanazirev, B. Prevalence of polypharmacy and risk of potential drug-drug interactions among hospitalized patients with emphasis on the pharmacokinetics. Sci. Prog. 2022, 105, 00368504211070183. [Google Scholar] [CrossRef]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. 2013, 18, 600–609. [Google Scholar]
- Hermann, M.; Carstens, N.; Kvinge, L.; Fjell, A.; Wennersberg, M.; Folleso, K.; Cronfalk, B.S. Polypharmacy and Potential Drug—Drug Interactions in Home-Dwelling Older People—A Cross-Sectional Study. J. Multidiscip. Healthc. 2021, 14, 589–597. [Google Scholar] [CrossRef]
- Wolff, J.; Hefner, G.; Normann, C.; Kaier, K.; Binder, H.; Hiemke, C.; Toto, S.; Domschke, K.; Marschollek, M.; Klimke, A. Polypharmacy and the risk of drug-drug interactions and potentially inappropriate medications in hospital psychiatry. Pharmacoepidemiol. Drug Saf. 2021, 30, 1258–1268. [Google Scholar] [CrossRef]
- Peng, Y.; Cheng, Z.; Xie, F. Evaluation of Pharmacokinetic Drug-Drug Interactions: A Review of the Mechanisms, In Vitro and In Silico Approaches. Metabolites 2021, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Ragueneau-Majlessi, I. Strong Pharmacokinetic Drug-Drug Interactions With Drugs Approved by the US Food and Drug Administration in 2021: Mechanisms and Clinical Implications. Clin. Ther. 2022, 44, 1536–1544. [Google Scholar] [CrossRef]
- Kristensen, M.B. Drug interactions and clinical pharmacokinetics. Clin. Pharmacokinet. 1976, 1, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Kandulski, A.; Venerito, M. Proton-pump inhibitors: Understanding the complications and risks. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 697–710. [Google Scholar] [CrossRef]
- Cheer, S.M.; Prakash, A.; Faulds, D.; Lamb, H.M. Pantoprazole. Drugs 2003, 63, 101–132. [Google Scholar] [CrossRef] [PubMed]
- Jungnickel, P.W. Pantoprazole: A new proton pump inhibitor. Clin. Ther. 2000, 22, 1268–1293. [Google Scholar] [CrossRef]
- Cheer, S.M.; Prakash, A.; Faulds, D.; Lamb, H.M.; Adamek, R.J. Pantoprazole An Update of its Pharmacological Properties and Therapeutic Use in the Management of Acid-Related Disorders. Drugs 2003, 63, 101–133. [Google Scholar] [CrossRef]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy--A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, M.F.; Sarwar, M.H.; Sarwar, M. Deficiency of Vitamin B-Complex and Its Relation with Body Disorders. In B-Complex Vitamins; LeBlanc, J.G., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Kujovská Krčmová, L.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef]
- Pelluri, R.; Shafiya Begum, S.; Sowmya, N.; Jyothsna, M.; Prathyusha, S.; Divya, Y.; Poornachand, T.; Vivek Reddy, T.; Jhansirani, K.; Umamounika, B.; et al. A Case Report on Pantoprazole Induced VIT.B12 Deficiency. Indian J. Pharm. Pract. 2016, 9, 63–64. [Google Scholar] [CrossRef]
- Ashour, S.; Omar, S. A modified high-performance liquid chromatographic method for the analysis of pantoprazole sodium in pharmaceutical dosage forms using lansoprazole as internal standard. Arab. J. Chem. 2016, 9, S114–S119. [Google Scholar] [CrossRef] [Green Version]
- Chatzimichalakis, P.F.; Samanidou, V.F.; Verpoorte, R.; Papadoyannis, I.N. Development of a validated HPLC method for the determination of B-complex vitamins in pharmaceuticals and biological fluids after solid phase extraction. J. Sep. Sci. 2004, 27, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, B.; Du Prel, J.B.; Wachtlin, D.; Kwiecien, R.; Blettner, M. Fallzahlplanung in klinischen studien: Teil 13 der serie zur bewertung wissenschaftlicher publikationen. Dtsch. Arztebl. 2010, 107, 552–556. [Google Scholar] [CrossRef]
- Association, W.M. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Jahrb. für Wiss. Und Ethik 2009, 14, 233–238. [Google Scholar] [CrossRef]
- Krleza-Jerić, K.; Lemmens, T. 7th revision of the Declaration of Helsinki: Good news for the transparency of clinical trials. Croat. Med. J. 2009, 50, 105–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Boonsongrit, Y.; Mueller, B.W.; Mitrevej, A. Characterization of drug-chitosan interaction by 1H NMR, FTIR and isothermal titration calorimetry. Eur. J. Pharm. Biopharm. 2008, 69, 388–395. [Google Scholar] [CrossRef]
- Pandey, M.M.; Jaipal, A.; Charde, S.Y.; Goel, P.; Kumar, L. Dissolution enhancement of felodipine by amorphous nanodispersions using an amphiphilic polymer: Insight into the role of drug–polymer interactions on drug dissolution. Pharm. Dev. Technol. 2015, 21, 463–474. [Google Scholar] [CrossRef]
- Emami, J.; Rezazadeh, M.; Kalani, M. Quantification of pantoprazole by high performance liquid chromatography (HPLC) method: In vitro and in vivo applications. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 681–695. [Google Scholar] [CrossRef]
- El Zaher, A.A.; El Kady, E.F.; EL Messiry, H.M.; El Ghwas, H.E.; El Houssini, O.M. Synchronous LC-MS/MS determination of pantoprazole and amitriptyline in rabbit plasma: Application to comparative in vivo pharmacokinetic study of novel formulated effervescent granules with its marketed tablet dosage form. Heliyon 2021, 7, e07752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, W.E.; Yan, J.Q.; Liu, M.; Zhou, Y.; Shen, X.; Ma, Y.L.; Feng, X.S.; Yang, J.; Li, G.H. A review of the extraction and determination methods of thirteen essential vitamins to the human body: An update from 2010. Molecules 2018, 23, 1484. [Google Scholar] [CrossRef] [Green Version]
- Zendelovska, D.; Atanasovska, E.; Gjorgjievska, K.; Pavlovska, K.; Jakjovski, K.; Zafirov, D.; Trojacanec, J. A new solid-phase extraction method for determination of pantoprazole in human plasma using high-performance liquid chromatography. Open Access Maced. J. Med. Sci. 2019, 7, 1757–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puts, J.; de Groot, M.; Haex, M.; Jakobs, B. Simultaneous Determination of Underivatized Vitamin B1 and B6 in Whole Blood by Reversed Phase Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry. PLoS ONE 2015, 10, e0132018. [Google Scholar] [CrossRef] [PubMed]
- Booth, B.P.; Simon, W.C. Analytical method validation. In New Drug Development; CRC Press: Boca Raton, FL, USA, 2016; pp. 138–159. [Google Scholar] [CrossRef]
- Neodo, A.; Schulz, J.D.; Huwyler, J.; Keiser, J. In vitro and in vivo drug-drug interaction study of the effects of ivermectin and oxantel pamoate on tribendimidine. Antimicrob. Agents Chemother. 2019, 63, e00762-18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Reynolds, K.S.; Zhao, P.; Huang, S.M. Drug interactions evaluation: An integrated part of risk assessment of therapeutics. Toxicol. Appl. Pharmacol. 2010, 243, 134–145. [Google Scholar] [CrossRef]
- Castelli, M.C.; Wong, D.F.; Friedman, K.; Riley, M.G.I. Pharmacokinetics of Oral Cyanocobalamin Formulated With Sodium N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC): An Open-Label, Randomized, Single-Dose, Parallel-Group Study in Healthy Male Subjects. Clin. Ther. 2011, 33, 934–945. [Google Scholar] [CrossRef]
- Kahoun, D.; Fojtíková, P.; Vácha, F.; Čížková, M.; Vodička, R.; Nováková, E.; Hypša, V. Development and validation of an LC-MS/MS method for determination of B vitamins and some its derivatives in whole blood. PLoS ONE 2022, 17, e0271444. [Google Scholar] [CrossRef]
- Heidelbaugh, J.J. Proton pump inhibitors and risk of vitamin and mineral deficiency: Evidence and clinical implications. Ther. Adv. Drug Saf. 2013, 4, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA J. Am. Med. Assoc. 2013, 310, 2435–2442. [Google Scholar] [CrossRef] [Green Version]
- Wedemeyer, R.S.; Blume, H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: An update. Drug Saf. 2014, 37, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spechler, S.J. Proton Pump Inhibitors: What the Internist Needs to Know. Med. Clin. N. Am. 2019, 103, 1–14. [Google Scholar] [CrossRef] [PubMed]





| Group 1 | Group 2 | Group 3 | ||
|---|---|---|---|---|
| Age (years) | 23.00 ± 2.66 | 22.63 ± 3.61 | 21.66 ± 0.96 | |
| Weight (kg) | 64.90 ± 9.51 | 65.63 ± 12.11 | 66.50 ± 14.02 | |
| Height (cm) | 165.20 ± 9.29 | 165.18 ± 11.35 | 166.30 ± 1.07 | |
| BMI, (kg/m2) | 23.64 ± 1.62 | 23.67 ± 2.56 | 23.78 ± 2.95 | |
| Gender | Male | 8 | 6 | 8 |
| Female | 2 | 4 | 2 | |
| Peak Assignment | Peak Position (cm−1) | ||||||
|---|---|---|---|---|---|---|---|
| PNT | VTB1 | VTB6 | VTB12 | PM of PNT and VTB1 | PM of PNT and VTB6 | PM of PNT and VTB12 | |
| N-H | 3485.37 | 3321.42 | 3323.35 | 3373.50 | 3373.50 | ||
| O-H | 3136.25 | 3776.62 3431.36 | 3136.25 | 3155.54 | 3772.76 | ||
| N-H | 3369.64 | 3041.74 | 3242.34 | 2943.37 | 3379.29 | ||
| C-H | 3196.05 | 2881.65 | 3091.89 | 2926.01 | 2945.30 | 2845.00 | 2941.44 |
| C=O | 1589.34 | 1543.05 | 1593.20 | 1591.27 | 1589.34 | 1591.27 | |
| C-N | 1371.39 | 1359.82 | 1159.22 | 1381.03 | 1382.96 | 1377.17 | |
| C-O | 1118.71 | 1276.88 | 1029.99 | 1118.51 | 1118.71 | ||
| S=O | 1035.77 | 1037.70 | 1031.92 | 1031.92 | 1033.85 | ||
| Parameters | Variables | PNT | VTB1 | VTB6 | VTB12 | ||||
|---|---|---|---|---|---|---|---|---|---|
| RT | % Recovery | RT | % Recovery | RT | % Recovery | RT | % Recovery | ||
| Flow rate (mL/min) | 0.3 | 13.2 | 83.2 ± 5.34 | 5.1 | 113.25 ± 2.87 | 9.2 | 112.8 ± 1.98 | 6.5 | 68.05 ± 2.67 |
| 0.5 | 6.6 | 123.70 ± 8.87 | 3.5 | 83.45 ± 3.25 | 5.5 | 98.73 ± 2.30 | 4.3 | 107.44 ± 1.34 | |
| Mobile Phase | Acetonitrile | 7.5 | 123.70 ± 8.87 | 3.5 | 83.45 ± 3.25 | 3.5 | 98.73 ± 2.30 | 4.3 | 107.44 ± 1.34 |
| Methanol | ND | ND | ND | ND | ND | ND | ND | ND | |
| Column (µm) | 250 × 4.6 | 9.2 | 102.22 ± 3.43 | 3.2 | 77.45 ± 2.39 | ND | ND | ND | ND |
| 150 × 4.6 | 6.8 | 123.70 ± 8.87 | 2.7 | 83.45 ± 3.25 | 5.5 | 98.73 ± 2.30 | 3.8 | 107.44 ± 1.34 | |
| Wavelength (nm) | 270 | ND | ND | ND | ND | ND | ND | 4.2 | 65.44 ± 4.90 |
| 280 | 7.6 | 123.70 ± 8.87 | 3.2 | 83.45 ± 3.25 | 5.5 | 124.36 ± 2.30 | 4.5 | 107.44 ± 1.34 | |
| 305 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 505 | ND | ND | ND | ND | ND | ND | 4.4 | 125.67 ± 7.32 | |
| Column Tem. (°C) | 30 | 6.5 | 123.70 ± 8.87 | 3.2 | 98.37 ± 2.49 | 6.1 | 112.23 ± 5.65 | 4.2 | 107.44 ± 1.34 |
| 35 | 7.5 | 123.70 ± 8.87 | 3.4 | 83.45 ± 3.25 | 5.5 | 98.73 ± 2.30 | 4.1 | 107.44 ± 1.34 | |
| Parameter | PNT | VTB1 | VTB6 | VTB12 |
|---|---|---|---|---|
| Retention time (min) | 6.8 ± 0.2 | 2.7 ± 0.1 | 5.5 ± 0.2 | 3.8 ± 0.1 |
| Assay (%) | 104.13 ± 2.30 | 102.99 ± 2.56 | 114.36 ± 1.94 | 90.01 ± 1.56 |
| Peak height | 3539 ± 175.68 | 7308 ± 956.72 | 51,846 ± 1749.48 | 3993.33 ± 633.33 |
| No of theoretical plates | 547.66 ± 72.23 | 1214 ± 61.02 | 4360 ± 1480.81 | 1058 ± 249.52 |
| USP Tailing Factor | 0.79 ± 0.06 | 1.47 ± 0.27 | 0.81 ± 0.3 | 1.81 ± 0.07 |
| Capacity factor | 2.5 ± 1.18 | 0.71 ± 0.07 | 1.48 ± 0.50 | 0.79 ± 0.21 |
| LOD (ng/mL) | 0.50 | 0.59 | 15.34 | 0.04 |
| LOQ (ng/mL) | 1.50 | 1.78 | 69.34 | 0.10 |
| Sample | Spiked Analyte (µg/mL) | Intra Day | Inter Day | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± S.D | CV% | Accuracy (RE%) | Recovery (%) | Mean ± S.D | CV% | Accuracy (RE%) | Recovery (%) | ||
| PNT | 10 | 13.07 ± 0.98 | 7.53 | −10.78 | 114.70 | 13.12 ± 1.34 | 10.22 | −11.22 | 113.83 |
| 20 | 19.65 ± 0.72 | 3.62 | 3.67 | 98.45 | 17.93 ± 3.17 | 12.72 | 10.23 | 89.65 | |
| 30 | 27.36 ± 1.44 | 5.26 | 8.77 | 91.20 | 28.34 ± 0.99 | 3.49 | 5.53 | 94.56 | |
| VTB1 | 10 | 10.89 ± 1.48 | 13.69 | −8.92 | 108.90 | 9.53 ± 0.78 | 8.24 | 4.73 | 95.30 |
| 20 | 17.37 ± 0.78 | 4.50 | −2.45 | 86.85 | 15.51 ± 3.45 | 12.21 | 12.43 | 77.55 | |
| 30 | 25.73 ± 1.56 | 6.07 | 14.20 | 85.76 | 26.16 ± 1.96 | 7.38 | 11.28 | 87.20 | |
| VTB6 | 10 | 10.23 ± 1.34 | 2.33 | −2.33 | 102.30 | 11.27 ± 0.23 | 2.34 | 3.45 | 112.70 |
| 20 | 19.23 ± 0.024 | 1.44 | 6.22 | 96.15 | 18.54 ± 0.34 | 6.34 | 2.34 | 92.70 | |
| 30 | 29.34 ± 0.03 | 2.44 | 8.22 | 97.8 | 26.97 ± 4.76 | 13.54 | 7.23 | 89.90 | |
| VTB12 | 10 | 7.31 ± 0.50 | 6.84 | 14.80 | 73.15 | 8.28 ± 0.27 | 3.36 | 14.12 | 82.8 |
| 20 | 21.23 ± 3.56 | 6.09 | −14.18 | 106.15 | 26.19 ± 7.10 | 12.12 | −10.95 | 87.3 | |
| 30 | 30.45 ± 3.58 | 11.77 | −1.57 | 101.50 | 38.08 ± 3.16 | 9.38 | −10.24 | 112.33 | |
| Parameters | Unit | PNT (20 mg/kg, p.o.) | VTB1 (100 mg/kg, p.o.) | VTB6 (200 mg/kg, p.o.) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alone | Combination | p | Alone | Combination | p | Alone | Combination | p | ||
| Cmax | μg/mL | 0.95 ± 0.347 | 0.98 ± 0.295 | 0.949 | 1.63 ± 0.154 | 1.58 ± 0.053 | 0.742 | 20.66 ± 1.969 | 24.09 ± 3.179 | 0.410 |
| Tmax | h | 2.67 ± 0.333 | 2.67 ± 0.333 | >0.99 | 1.67 ± 0.333 | 2.33 ± 0.333 | 0.230 | 2.00 ± 1.20 | 2.67 ± 0.333 | 0.116 |
| AUC0−6 | μg/mL * h | 3.88 ± 1.239 | 3.56 ± 0.356 | 0.816 | 8.44 ± 0.514 | 7.90 ± 0.130 | 0.366 | 62.91 ± 3.046 | 56.52 ± 6.816 | 0.440 |
| AUC0−inf | μg/mL * h | 5.03 ± 0.950 | 4.73 ± 0.344 | 0.782 | 39.72 ± 10.417 | 51.37 ± 18.469 | 0.612 | 95.03 ± 29.349 | 60.61 ± 6.732 | 0.317 |
| MRT | h | 4.95 ± 0.993 | 4.71 ± 0.334 | 0.832 | 24.93 ± 8.890 | 34.83 ± 14.736 | 0.596 | 5.13 ± 1.882 | 3.36 ± 0.165 | 0.402 |
| CL | Lh−1 | 4.33 ± 0.731 | 4.27 ± 0.291 | 0.936 | 2.78 ± 0.601 | 2.44 ± 0.697 | 0.725 | 2.18 ± 0.914 | 3.16 ± 0.370 | 0.376 |
| Vd | L | 17.94 ± 6.516 | 16.78 ± 1.605 | 0.969 | 59.06 ± 6.071 | 63.60 ± 4.763 | 0.588 | 7.58 ± 3.021 | 9.71 ± 6.258 | 0.774 |
| t1/2 | h | 2.74 ± 0.827 | 2.72 ± 0.155 | 0.988 | 17.01 ± 6.177 | 23.85 ± 10.302 | 0.600 | 2.38 ± 1.200 | 1.18 ± 0.442 | 0.401 |
| Ke | h−1 | 0.32 ± 0.120 | 0.26 ± 0.014 | 0.609 | 0.05 ± 0.016 | 0.04 ± 0.014 | 0.610 | 0.45 ± 0.169 | 0.75 ± 0.229 | 0.354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanam, S.; Halder, S.; Rahman, S.M.A. Simultaneous Pharmacokinetic Evaluation of Pantoprazole and Vitamin B Complex for Assessing Drug–Drug Interactions in Healthy Bangladeshi Adults by a Newly Developed and Validated HPLC Method. Separations 2023, 10, 170. https://doi.org/10.3390/separations10030170
Sanam S, Halder S, Rahman SMA. Simultaneous Pharmacokinetic Evaluation of Pantoprazole and Vitamin B Complex for Assessing Drug–Drug Interactions in Healthy Bangladeshi Adults by a Newly Developed and Validated HPLC Method. Separations. 2023; 10(3):170. https://doi.org/10.3390/separations10030170
Chicago/Turabian StyleSanam, Sherejad, Shimul Halder, and S. M. Abdur Rahman. 2023. "Simultaneous Pharmacokinetic Evaluation of Pantoprazole and Vitamin B Complex for Assessing Drug–Drug Interactions in Healthy Bangladeshi Adults by a Newly Developed and Validated HPLC Method" Separations 10, no. 3: 170. https://doi.org/10.3390/separations10030170
APA StyleSanam, S., Halder, S., & Rahman, S. M. A. (2023). Simultaneous Pharmacokinetic Evaluation of Pantoprazole and Vitamin B Complex for Assessing Drug–Drug Interactions in Healthy Bangladeshi Adults by a Newly Developed and Validated HPLC Method. Separations, 10(3), 170. https://doi.org/10.3390/separations10030170