Development of the QuEChERS Extraction Method for the Determination of Polychlorinated Biphenyls (Aroclor 1254) in Soil Samples by Using GC-MS
Abstract
:1. Introduction
2. Experiment
2.1. Instrumentation and Conditions for GC-MS
2.2. Chemicals and Instruments
2.3. Standard and Sample Solution Preparation
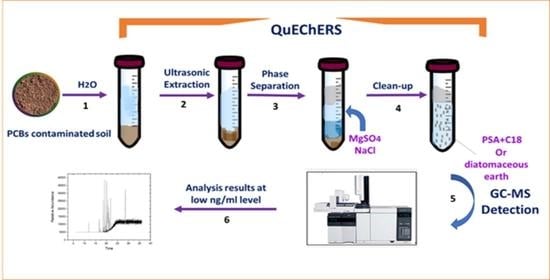
2.4. Extraction and Clean-Up Procedure
2.5. Method Validation
3. Results and Discussion
3.1. Optimization of Extraction Solvent Conditions
3.2. Optimization of Physical Extraction Condition
3.3. Method Validation
3.4. Analysis of Aroclor 1254 in Spiked Soil Samples (Recovery and Precision)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robles Martínez, H.A.; Rodríguez, G.C.; Castillo, D.H. Determination of PCBs in Transformers Oil Using Gas Chromatography with Mass Spectroscopy and Aroclors (A1254:A1260). Chem. Soc. 2005, 49, 263–270. [Google Scholar]
- Shin, S.K.; Kim, T.S. Levels of polychlorinated biphenyls (PCBs) in transformer oils from Korea. J. Hazard. Mater. 2006, 137, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Xu, Y.J.; Wang, Y.; Zhu, P.Y.; Dai, X.L.; Chen, J.; Zou, Q. Determination of Aroclor 1254 in Water Samples Using Polystyrene–Divinylbenzene Solid-Phase Extraction and Gas Chromatography. J. Anal. Test. 2018, 2, 306–311. [Google Scholar] [CrossRef]
- Kim, L.; Jeon, J.W.; Lee, Y.S.; Jeon, H.J.; Park, B.J.; Lee, H.S.; Choi, S.D.; Lee, S.E. Monitoring and risk assessment of polychlorinated biphenyls (PCBs) in agricultural soil collected in the vicinity of an industrialized area. Appl. Biol. Chem. 2016, 59, 655–659. [Google Scholar] [CrossRef]
- Kaya, D.; Imamoglu, I.; Sanin, F.D.; Payne, R.B.; Sowers, K.R. Potential risk reduction of Aroclor 1254 by microbial dechlorination in anaerobic Grasse River sediment microcosms. J. Hazard. Mater. 2017, 321, 879–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, G.M.; Ratcliffe, H.E.; Fischer, L.J. Human Exposure to Polychlorinated Biphenyls (PCBs): A Critical Assessment of the Evidence for Adverse Health Effects. Regul. Toxicol. Pharmacol. 1995, 21, 136–150. [Google Scholar] [CrossRef]
- Zhong, Y.; Guo, P.; Wang, X.; An, J. Aroclor 1254 inhibits cell viability and induces apoptosis of human A549 lung cancer cells by modulating the intracellular Ca2+ level and ROS production through the mitochondrial pathway. J. Environ. Sci. Health Part A 2015, 50, 806–813. [Google Scholar] [CrossRef]
- Ozcan, S. Analyses of polychlorinated biphenyls in waters and wastewaters using vortex-assisted liquid-liquid microextraction and gas chromatography-mass spectrometry. J. Sep. Sci. 2011, 34, 574–584. [Google Scholar] [CrossRef]
- Ozcan, S.; Tor, A.; Aydin, M.E. Determination of selected polychlorinated biphenyls in water samples by ultrasound-assisted emulsification-microextraction and gas chromatography-mass-selective detection. Anal. Chim. Acta 2009, 647, 182–188. [Google Scholar] [CrossRef]
- Derouiche, A.; Driss, M.R.; Morizur, J.P.; Taphanel, M.H. Simultaneous analysis of polychlorinated biphenyls and organochlorine pesticides in water by headspace solid-phase microextraction with gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1138, 231–243. [Google Scholar] [CrossRef]
- Güzel, B.; Canlı, O. An environmental friendly and stable analytical method for the determination of indicator polychlorinated biphenyls (PCBs) in solid and waste oil samples by gas chromatography-electron capture detector (GC-ECD). Microchem. J. 2022, 178, 107325. [Google Scholar] [CrossRef]
- Santos, F.J.; Galceran, M.T. The application of gas chromatography to environmental analysis. TrAC—Trends Anal. Chem. 2002, 21, 672–685. [Google Scholar] [CrossRef]
- Bandh, C.; Björklund, E.; Mathiasson, L.; Näf, C.; Zebühr, Y. Comparison of accelerated solvent extraction and Soxhlet extraction for the determination of PCBs in Baltic Sea sediments. Environ. Sci. Technol. 2000, 34, 4995–5000. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Wang, Y.; Wang, T.; Li, X.; Ding, L.; Jiang, G. Evaluation of Soxhlet extraction, accelerated solvent extraction and microwave-assisted extraction for the determination of polychlorinated biphenyls and polybrominated diphenyl ethers in soil and fish samples. Anal. Chim. Acta 2010, 663, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Luque-García, J.L.; Luque De Castro, M.D. Extraction of polychlorinated biphenyls from soils by automated focused microwave-assisted Soxhlet extraction. J. Chromatogr. A 2003, 998, 21–29. [Google Scholar] [CrossRef]
- Barri, T.; Bergström, S.; Norberg, J.; Jönsson, J.Å. Miniaturized and Automated Sample Pretreatment for Determination of PCBs in Environmental Aqueous Samples Using an On-Line Microporous Membrane Liquid-Liquid Extraction-Gas Chromatography System. Anal. Chem. 2004, 76, 1928–1934. [Google Scholar] [CrossRef]
- Rezaei, F.; Bidari, A.; Birjandi, A.P.; Milani Hosseini, M.R.; Assadi, Y. Development of a dispersive liquid-liquid microextraction method for the determination of polychlorinated biphenyls in water. J. Hazard. Mater. 2008, 158, 621–627. [Google Scholar] [CrossRef]
- Westbom, R.; Thörneby, L.; Zorita, S.; Mathiasson, L.; Björklund, E. Development of a solid-phase extraction method for the determination of polychlorinated biphenyls in water. J. Chromatogr. A 2004, 1033, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Rhind, S.M. Optimized determination of polybrominated diphenyl ethers and polychlorinated biphenyls in sheep serum by solid-phase extraction-gas chromatography-mass spectrometry. Talanta 2011, 84, 487–493. [Google Scholar] [CrossRef]
- Huo, L.G.; Li, H.D.; Zhao, C.L.; Wang, W.B.; Chen, Z.L.; Ding, R.Y.; Dong, Z.; Wang, F.E.; Yang, G.S.; Lu, X.; et al. The Determination of PCBs in Meat and Sea Food by GC-QqQ-MS/MS. Food Anal. Methods 2012, 5, 1481–1491. [Google Scholar] [CrossRef]
- Ecd, G.C.; Benedicto, J.; Beckert, F. Determination of PCBs in Soils / Sediments by Microwave-Assisted Extraction. Environ. Sci. Technol. 1995, 29, 2709–2712. [Google Scholar]
- Reddy, A.V.B.; Moniruzzaman, M.; Aminabhavi, T.M. Polychlorinated biphenyls (PCBs) in the environment: Recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chem. Eng. J. 2019, 358, 1186–1207. [Google Scholar] [CrossRef]
- Camel, V. Recent extraction techniques for solid matrices—Supercritical fluid extraction, pressurized fluid extraction and microwave-assisted extraction: Their potential and pitfalls. Analyst 2001, 126, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- Chamkasem, N.; Lee, S.; Harmon, T. Analysis of 19 PCB congeners in catfish tissue using a modified QuEChERS method with GC-MS/MS. Food Chem. 2016, 192, 900–906. [Google Scholar] [CrossRef]
- Norli, H.R.; Christiansen, A.; Deribe, E. Application of QuEChERS method for extraction of selected persistent organic pollutants in fish tissue and analysis by gas chromatography mass spectrometry. J. Chromatogr. A 2011, 1218, 7234–7241. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Z.; Cong, J.; Wang, L.; Liu, Y.; Wang, X.; Xie, J. Determination of polychlorinated biphenyls in fish tissues from Shanghai seafood markets using a modified QuEChERS method. Anal. Sci. 2017, 33, 973–977. [Google Scholar] [CrossRef] [Green Version]
- Madureira, T.V.; Santos, C.; Velhote, S.; Cruzeiro, C.; Rocha, E.; Rocha, M.J. Contamination levels of polychlorinated biphenyls in wild versus cultivated samples of female and male mussels (Mytilus sp.) from the Northwest Coast of Iberian Peninsula-new application for QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) metho. Environ. Sci. Pollut. Res. 2014, 21, 1528–1540. [Google Scholar] [CrossRef]
- Kuzukiran, O.; Filazi, A. Determination of Selected Polychlorinated Biphenyl Residues in Meat Products by QuEChERS Method Coupled with Gas Chromatography–Mass Spectrometry. Food Anal. Methods 2016, 9, 1867–1875. [Google Scholar] [CrossRef]
- Al-Alam, J.; Fajloun, Z.; Chbani, A.; Millet, M. A multiresidue method for the analysis of 90 pesticides, 16 PAHs, and 22 PCBs in honey using QuEChERS–SPME. Anal. Bioanal. Chem. 2017, 409, 5157–5169. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Domingues, V.F.; Mateus, N.; Delerue-Matos, C. Multiresidue pesticides analysis in soils using modified QuEChERS with disposable pipette extraction and dispersive solid-phase extraction. J. Sep. Sci. 2013, 36, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Liu, X.; He, Z.; Wang, L.; Luo, M.; Peng, Y.; Zhou, Q. Development of a multi-residue method for 58 pesticides in soil using QuEChERS and gas chromatography-tandem mass spectrometry. Anal. Methods 2016, 8, 2463–2470. [Google Scholar] [CrossRef]
- Rouvire, F.; Buleté, A.; Cren-Olivé, C.; Arnaudguilhem, C. Multiresidue analysis of aromatic organochlorines in soil by gas chromatography-mass spectrometry and QuEChERS extraction based on water/dichloromethane partitioning. Comparison with accelerated solvent extraction. Talanta 2012, 93, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Robert Baudot, L.W. Comparison of Two Analytical Methods for the Determination of Traces of Veterinary Antibiotics and Steroid Hormones in Soil Based on Pressurised Liquid Extraction (PLE) and Quick, Easy, Cheap, Effective, Rugged, Safe (Modified-Quechers) Extraction. Pharm. Anal. Acta 2014, 5, 1000315. [Google Scholar] [CrossRef] [Green Version]
- De Mastro, F.; Cocozza, C.; Traversa, A.; Cacace, C.; Mottola, F.; Mezzina, A.; Brunetti, G. Validation of a modified QuEChERS method for the extraction of multiple classes of pharmaceuticals from soils. Chem. Biol. Technol. Agric. 2022, 9, 49. [Google Scholar] [CrossRef]
- Lacina, O.; Zachariasova, M.; Urbanova, J.; Vaclavikova, M.; Cajka, T.; Hajslova, J. Critical assessment of extraction methods for the simultaneous determination of pesticide residues and mycotoxins in fruits, cereals, spices and oil seeds employing ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1262, 8–18. [Google Scholar] [CrossRef]
- Walorczyk, S. Development of a multi-residue screening method for the determination of pesticides in cereals and dry animal feed using gas chromatography-triple quadrupole tandem mass spectrometry. J. Chromatogr. A 2007, 1165, 200–212. [Google Scholar] [CrossRef]
- Cajka, T.; Sandy, C.; Bachanova, V.; Drabova, L.; Kalachova, K.; Pulkrabova, J.; Hajslova, J. Streamlining sample preparation and gas chromatography-tandem mass spectrometry analysis of multiple pesticide residues in tea. Anal. Chim. Acta 2012, 743, 51–60. [Google Scholar] [CrossRef]
- Salama, G.; El Gindy, A.; Hameed, E.A.A. The use of experimental design for optimisation of QuEChERS extraction of commonly used pesticides in Egyptian soil and drainage water and their determination by GC/MS. Int. J. Environ. Anal. Chem. 2022, 102, 4238–4249. [Google Scholar] [CrossRef]
- Salimiasl, S.M.; Khodayar, M.J.; Mousavi, Z.; Akhgari, M. Comparison of the Modified QuEChERS Method and the Conventional Method of Extraction in Forensic Medicine to Detect Methadone in Post-Mortem Urine by GCMS. Asia Pac. J. Med. Toxicol. 2017, 6, 81–87. [Google Scholar]
- Zhang, C.; Deng, Y.; Zheng, J.; Zhang, Y.; Yang, L.; Liao, C.; Su, L.; Zhou, Y.; Gong, D.; Chen, L.; et al. The application of the QuEChERS methodology in the determination of antibiotics in food: A review. TrAC—Trends Anal. Chem. 2019, 118, 517–537. [Google Scholar] [CrossRef]
- Szostek, B.; Tinklenberg, J.A.; Aldstadt, J.H. A simple method for the quantitative microextraction of polychlorinated biphenyls from soils and sediments. Chemosphere 1999, 38, 3131–3139. [Google Scholar] [CrossRef]
- Pajewska-Szmyt, M.; Sinkiewicz-Darol, E.; Bernatowicz-Łojko, U.; Kowalkowski, T.; Gadzała-Kopciuch, R.; Buszewski, B. QuEChERS extraction coupled to GC-MS for a fast determination of polychlorinated biphenyls in breast milk from Polish women. Environ. Sci. Pollut. Res. 2019, 26, 30988–30999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Wang, L.; Peng, Y.; Luo, M.; Wang, W.; Liu, X. Determination of selected polychlorinated biphenyls in soil and earthworm (Eisenia fetida) using a QuEChERS-based method and gas chromatography with tandem MS. J. Sep. Sci. 2015, 38, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Núñez, M.; Borrull, F.; Pocurull, E.; Fontanals, N. Sample treatment for the determination of emerging organic contaminants in aquatic organisms. TrAC—Trends Anal. Chem. 2017, 97, 136–145. [Google Scholar] [CrossRef]
- Al-Thaiban, H.; Al-Tamimi, N.; Helaleh, M. Development of QuEChERS method for the determination of polycyclic aromatic hydrocarbons in smoked meat products using GC-MS from Qatar. J. Anal. Methods Chem. 2018, 2018, 9206237. [Google Scholar] [CrossRef]
- Vera, J.; Correia-Sá, L.; Paíga, P.; Bragança, I.; Fernandes, V.C.; Domingues, V.F.; Delerue-Matos, C. QuEChERS and soil analysis. An Overview. Sample Prep. 2014, 1, 54–77. [Google Scholar] [CrossRef]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M.; et al. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018, 613–614, 361–370. [Google Scholar] [CrossRef]
- Kouzayha, A.; Al Iskandarani, M.; Mokh, S.; Rabaa, A.R.; Budzinski, H.; Jaber, F. Optimization of a solid-phase extraction method using centrifugation for the determination of 16 polycyclic aromatic hydrocarbons in water. J. Agric. Food Chem. 2011, 59, 7592–7600. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Sohrabi, H.; Mohebbi, A. Combination of Modified QuEChERS Extraction Method and Dispersive Liquid–Liquid Microextraction as an Efficient Sample Preparation Approach for Extraction and Preconcentration of Pesticides from Fruit and Vegetable Samples. Food Anal. Methods 2019, 12, 534–543. [Google Scholar] [CrossRef]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24, 7124–7138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Arias, J.L.O.; Rombaldi, C.; Caldas, S.S.; Primel, E.G. Alternative sorbents for the dispersive solid-phase extraction step in quick, easy, cheap, effective, rugged and safe method for extraction of pesticides from rice paddy soils with determination by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1360, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, L.D.C.; Caldas, S.S.; Prestes, O.D.; Primel, E.G.; Zanella, R. Evaluation of alternative sorbents for dispersive solid-phase extraction clean-up in the QuEChERS method for the determination of pesticide residues in rice by liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-W.; Zhao, Y.-G.; Fu, Z.-J.; Li, J.-G.; Wang, Y.-F.; Yang, T.-C. Development of a Screening Method for the Determination of PCBs in Water Using QuEChERS Extraction and Gas Chromatography–Triple Quadrupole Mass Spectrometry. Anal. Sci. 2012, 28, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Sapozhnikova, Y.; Lehotay, S.J. Streamlined sample cleanup using combined dispersive solid-phase extraction and in-vial filtration for analysis of pesticides and environmental pollutants in shrimp. Anal. Chim. Acta 2014, 827, 40–46. [Google Scholar] [CrossRef]
- Ben Salem, F.; Ben Said, O.; Duran, R.; Monperrus, M. Validation of an adapted QuEChERS method for the simultaneous analysis of polycyclic aromatic hydrocarbons, polychlorinated biphenyls and organochlorine pesticides in sediment by gas chromatography–mass spectrometry. Bull. Environ. Contam. Toxicol. 2016, 96, 678–684. [Google Scholar] [CrossRef]





| Sample | Extraction | Added (µg/kg) | Intra-Day (RSD%) | Inter-Day (RSD%) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|
| Water | acetonitrile | 8 /L) | ≥15% | - | 90–95% | [54] |
| Shrimp | water/MeCN * | 1, 5, 10 | >20% | - | 70–115% | [55] |
| Sedimen | water/Ace/Hex * or wter/Ace/DCM * | 50 | ≥15% | - | 76–131% | [56] |
| Soil | Water/acetonitrile | 5 | 4 | 5.8 | 103.2% | Present work |
| Soil | water/acetonitrile | 10 | 2.1 | 3.6 | 95.3% | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsefri, S.; Balbaied, T.; Alatawi, H.; Albalawi, I.; Hogan, A.; Moore, E. Development of the QuEChERS Extraction Method for the Determination of Polychlorinated Biphenyls (Aroclor 1254) in Soil Samples by Using GC-MS. Separations 2023, 10, 250. https://doi.org/10.3390/separations10040250
Alsefri S, Balbaied T, Alatawi H, Albalawi I, Hogan A, Moore E. Development of the QuEChERS Extraction Method for the Determination of Polychlorinated Biphenyls (Aroclor 1254) in Soil Samples by Using GC-MS. Separations. 2023; 10(4):250. https://doi.org/10.3390/separations10040250
Chicago/Turabian StyleAlsefri, Samia, Thanih Balbaied, Hanan Alatawi, Ibtihaj Albalawi, Anna Hogan, and Eric Moore. 2023. "Development of the QuEChERS Extraction Method for the Determination of Polychlorinated Biphenyls (Aroclor 1254) in Soil Samples by Using GC-MS" Separations 10, no. 4: 250. https://doi.org/10.3390/separations10040250
APA StyleAlsefri, S., Balbaied, T., Alatawi, H., Albalawi, I., Hogan, A., & Moore, E. (2023). Development of the QuEChERS Extraction Method for the Determination of Polychlorinated Biphenyls (Aroclor 1254) in Soil Samples by Using GC-MS. Separations, 10(4), 250. https://doi.org/10.3390/separations10040250