Separation of Co(II) and Ni(II) Using an Analog of Glycine-Betaine Based on Task-Specific Ionic Liquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ionic Liquid Synthesis
2.3. Extraction Experiments
2.4. Determination of Organic Cation Concentration in the Aqueous Phase
2.5. Determination of Water Concentration in the Organic Phase after Extraction and Stripping
3. Results and Discussion
3.1. Synthesis
3.2. Physical Properties
3.2.1. Thermal Properties
3.2.2. Solubility of Ionic Liquids in Water and Salt Media
3.3. Co(II) and Ni(II) Extraction and Stripping in Saline Media
3.3.1. Co(II) Extraction Kinetic
3.3.2. Co(II) Extraction in Saline Media
- (a)
- Influence of the solvent and salt concentration
- (b)
- Influence of the coordinating anion of the ionic liquid
- (c)
- Extraction mechanism of Co(II) by [Pn3NC2OC2][Dca]
- (d)
- Co(II) stripping
3.3.3. Ni(II) Extraction in Saline Media
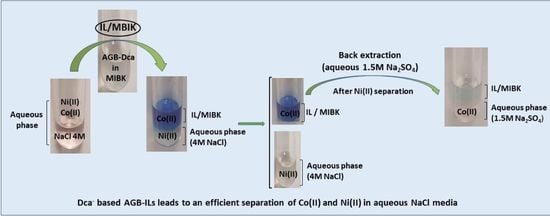
3.3.4. Co(II)/Ni(II) Extraction in Saline Media
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Lecce, D.; Marangon, V.; Jung, H.-G.; Tominaga, Y.; Greenbaum, S.; Hassoun, J. Glyme-based electrolytes: Suitable solutions for next-generation lithium batteries. Green Chem. 2022, 24, 1021–1048. [Google Scholar] [CrossRef]
- Cheng, Q.; Marchetti, B.; Chen, X.; Xu, S.; Zhou, X.-D. Separation, purification, regeneration and utilization of graphite recovered from spent lithium-ion batteries—A review. J. Environ. Chem. Eng. 2022, 10, 10. [Google Scholar] [CrossRef]
- Larsson, K.; Binnemans, K. Selective extraction of metals using ionic liquids for nickel metal hydride battery recycling. Green Chem. 2014, 16, 4595–4603. [Google Scholar] [CrossRef]
- European Commission. Study on the Review of the List of Critical Raw Materials; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.-M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Jha, R.; Meshram, A.; Singh, K.K. A review on recycling of lithium-ion batteries to recover critical metals. J. Environ. Chem. Eng. 2022, 10, 108534. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Ichlas, Z.T.; Kaya, M. Solvent extraction process for the recovery of nickel and cobalt from Caldag laterite leach solution: The first bench scale study. Hydrometallurgy 2017, 169, 135–141. [Google Scholar] [CrossRef]
- Cheng, C.Y. Purification of synthetic laterite leach solution by solvent extraction using D2EHPA. Hydrometallurgy 2000, 56, 369–386. [Google Scholar] [CrossRef]
- Park, K.H.; Reddy, B.R.; Jung, S.H.; Mohapatra, D. Transfer of cobalt and nickel from sulphate solutions to spent electrolyte through solvent extraction and stripping. Sep. Purif. Technol. 2006, 51, 265–271. [Google Scholar] [CrossRef]
- Sayar, N.A.; Filiz, M.; Sayar, A.A. Extraction of Co(II) and Ni(II) from concentrated HCl solutions using Alamine 336. Hydrometallurgy 2009, 96, 148–153. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, M. Separation of Co and Ni from a chloride leach solutions of laterite ore by solvent extraction with extractant mixtures. J. Ind. Eng. Chem. 2015, 28, 322–327. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Rogers, R.D. Room temperature ionic liquids as novel media for ‘clean’ liquid–liquid extraction. Chem. Commun. 1998, 16, 1765–1766. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as ‘green’ solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Visser, E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J.H., Jr.; Rogers, R.D. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem. Commun. 2001, 135–136. [Google Scholar] [CrossRef]
- Zhang, L.; Hessel, V.; Peng, J. Liquid-liquid extraction for the separation of Co(II) from Ni(II) with Cyanex 272 using a pilot scale Re-entrance flow microreactor. Chem. Eng. J. 2018, 332, 131–139. [Google Scholar] [CrossRef]
- Coll, M.T.; Fortuny, A.; Kedari, C.S.; Sastre, A.M. Studies on the extraction of Co(II) and Ni(II) from aqueous chloride solutions using Primene JMT-Cyanex272 ionic liquid extractant. Hydrometallurgy 2012, 125–126, 24–28. [Google Scholar] [CrossRef]
- Cholico-Gonzalez, D.; Chagnes, A.; Cote, G.; Avila-Rodriguez, M. Separation of Co(II) and Ni(II) from aqueous solutions by bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272) using trihexyl(tetradecyl)phosphonium chloride (Cyphos IL 101) as solvent. J. Mol. Liq. 2015, 209, 203–208. [Google Scholar] [CrossRef]
- de Lemos, L.R.; Santos, I.J.B.; Rodrigues, G.D.; da Silva, L.H.M.; da Silva, M.C.H. Copper recovery from ore by liquid–liquid extraction using aqueous two-phase, system. J. Hazard. Mat. 2012, 237–238, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Papaiconomou, N.; Lee, J.M.; Salminen, J.; Von Stosch, M.; Prausnitz, J.M.; John, M. Selective extraction of copper, mercury, silver, and palladium ions from water using hydrophobic ionic liquids. Ind. Eng. Chem. Res. 2008, 47, 5080–5086. [Google Scholar] [CrossRef]
- De los Rios, A.P.; Hernandez-Fernandez, F.J.; Lozano, L.J.; Sanchez, S.; Moreno, J.I.; Godinez, C. Removal of metal ions from aqueous solutions by extraction with ionic liquids. J. Chem. Eng. Data 2010, 55, 605–608. [Google Scholar] [CrossRef]
- Messadi, A.; Mohamadou, A.; Boudesocque, S.; Dupont, L.; Guillon, E. Task-specific ionic liquid with coordinating anion for heavy metal ion extraction: Cation ex-change versus ion-pair extraction. Sep. Purif. Technol. 2013, 107, 172–178. [Google Scholar] [CrossRef]
- Boudesocque, S.; Mohamadou, A.; Martinez, A.; Dechamps, I.; Dupont, L. Use of dicyanamide ionic liquids for metal ions extraction. RSC Adv. 2016, 6, 107894–107904. [Google Scholar] [CrossRef]
- Diabate, P.D.; Dupont, L.; Boudesocque, S.; Mohamadou, A. Novel task specific ionic liquids to remove heavy and noble metals from aqueous solutions. Metals 2018, 8, 412. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Filipowiak, K.; Wojciechowska, I.; Aksamitowski, P. Novel ionic liquid-modified polymers for highly effective adsorption of heavy metals ions. Sep. Purif. Technol. 2020, 236, 116313. [Google Scholar] [CrossRef]
- Whitehead, J.A.; Lawrence, G.A.; Mc Clusquet, A. ‘Green’ leaching: Recyclable and selective leaching of gold-bearing ore in an ionic liquid. Green Chem. 2004, 6, 313–315. [Google Scholar] [CrossRef]
- Whitehead, J.A.; Zhang, J.; Pereira, N.; Mc Cluskey, A.; Lawrance, G.A. Application of 1-alkyl-3-methyl-imidazolium ionic liquids in the oxidative leaching of sulphidic copper, gold and silver ores. Hydrometallurgy 2007, 88, 109–120. [Google Scholar] [CrossRef]
- Lee, J.M. Extraction of noble metal ions from aqueous solution by ionic liquids. Fluid Phase Equilib. 2012, 319, 30–36. [Google Scholar] [CrossRef]
- Tong, Y.; Yang, H.; Li, J.; Yang, Y. Extraction of Au(III) by ionic liquid from hydrochloric acid medium. Sep. Purif. Technol. 2013, 120, 367–372. [Google Scholar] [CrossRef]
- Papaiconomou, N.; Génand-Pinaz, S.; Leveque, J.M.; Guittoneau, S. Selective extraction of gold and platinum in water using ionic liquids a simple two-step extraction process. Dalton. Trans. 2013, 42, 979–1982. [Google Scholar] [CrossRef]
- Papaiconomou, N.; Vite, G.; Leveque, J.M.; Billard, I. Efficient removal of gold complexes from water by precipitation or liquid–liquid extraction using ionic liquids. Green Chem. 2012, 14, 2050–2056. [Google Scholar] [CrossRef]
- Génand-Pinaz, S.; Papaiconomou, N.; Leveque, J.M. Removal of platinum from water by precipitation or liquid–liquid extraction and separation from gold using ionic liquids. Green Chem. 2013, 15, 2493–2501. [Google Scholar] [CrossRef]
- Boudesocque, S.; Mohamadou, A.; Dupont, L. Efficient extraction of gold from water by liquid–liquid extraction or precipitation using hydrophobic ionic liquids. New J. Chem. 2014, 38, 5573–5581. [Google Scholar] [CrossRef]
- Boudesocque, S.; Mohamadou, A.; Conreux, A.; Marin, B.; Dupont, L. The recovery and selective extraction of gold and platinum by novel ionic liquids. Sep. Purif. Technol. 2019, 210, 824–834. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Lee, J.; Jeong, J.; Kim, B.; Cote, G.; Chagnes, A. Extraction of Gold(III) from Acidic Chloride Media Using Phosphonium-based Ionic Liquid as an Anion Exchanger. Ind. Eng. Chem. Res. 2015, 54, 1350–1358. [Google Scholar] [CrossRef]
- Jensen, M.P.; Dzielawa, J.A.; Rickert, P.; Dietz, M.L. EXAFS Investigations of the mechanism of facilitated ion transfer into a room temperature ionic liquid. J. Am. Chem. Soc. 2002, 124, 10664–10665. [Google Scholar] [CrossRef]
- Dietz, M.L.; Dzielawa, J.A. Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers: Implications for the ‘greenness’ of ionic liquids as diluents in liquid–liquid extraction. Chem. Commun. 2001, 20, 2124–2125. [Google Scholar] [CrossRef]
- Dietz, M.L.; Dzielawa, J.A.; Laszak, I.; Young, B.A.; Jensen, M.P. Influence of solvent structural variations on the mechanism of facilitated ion transfer into room-temperature ionic liquids. Green Chem. 2003, 6, 682–685. [Google Scholar] [CrossRef]
- Dietz, M.L.; Stepinski, D.C. A ternary mechanism for the facilitated transfer of metal ions into room-temperature ionic liquids (RTILs): Implications for the “greenness” of RTILs as extraction solvent. Green Chem. 2005, 7, 747–750. [Google Scholar] [CrossRef]
- Parmentier, D.; Hoogerstraete, T.V.; Metz, S.J.; Binnemans, K.; Kroon, M.C. Selective extraction of metals from chloride solutions with the tetraoctylphosphonium oleate ionic liquid. Ind. Eng Chem. Res. 2015, 54, 5149–5158. [Google Scholar] [CrossRef]
- Parmentier, D.; Hoogerstraete, T.V.; Banerjee, D.; Valia, Y.A.; Metz, S.J.; Binnemans, K.; Kroon, M.C. A mechanism for solvent extraction of first row transition metals from chloride media with the ionic liquid tetraoctylammonium oleate. Dalton. Trans. 2016, 45, 9661–9668. [Google Scholar] [CrossRef]
- Gras, M.; Papaiconomou, N.; Schaeffer, N.; Chainet, E.; Tedjar, F.; Coutinho, J.A.P.; Billard, I. Ionic-Liquid-Based Acidic Aqueous Biphasic Systems for Simultaneous Leaching and Extraction of Metallic Ions. Angew. Chem. Int. Ed. 2018, 57, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Tatarczak-Michalewska, M.; Blicharskaa, E.; Madejskaa, A.; Fliegerc, W.; Adamczukc, A. Extraction of cobalt (II) using ionic liquid-based bi-phase and three-phase systems without adding any chelating agents with new recycling procedure. Sep. Purif. Technol. 2019, 209, 984–989. [Google Scholar] [CrossRef]
- Schaeffer, N.; Gras, M.; Passos, H.; Mojilireddy, V.; Mendoça, C.M.N.; Pereira, E.; Chainet, E.; Tedjar, F.; Coutinho, J.A.P.; Billard, I.; et al. Synergistic Aqueous Biphasic Systems: A New Paradigm for the “One-Pot” Hydrometallurgical Recovery of Critical Metals. ACS Sustain. Chem. Eng. 2019, 7, 1769–1777. [Google Scholar] [CrossRef]
- Depuydt, D.; Van den Bossche, A.; Dehaen, W.; Binnemans, K. Metal extraction with a short-chain imidazolium nitrate ionic liquid. Chem. Commun. 2017, 38, 5271–5274. [Google Scholar] [CrossRef]
- Foltova, S.S.; Hoogerstraete, T.V.; Banerjee, D.; Binnemans, K. Samarium/cobalt separation by solvent extraction with undiluted quaternary ammonium ionic liquids. Sep. Purif. Technol. 2019, 210, 209–218. [Google Scholar] [CrossRef]
- Janssen, C.H.C.; Macias-Ruvalcaba, N.A.; Aguilar-Martinez, M.; Kobrak, M.N. Copper extraction using protic ionic liquids: Evidence of the Hofmeister effect. Sep. Purif. Technol. 2016, 168, 275–283. [Google Scholar] [CrossRef]
- Diabate, P.D.; Boudesocque, S.; Mohamadou, A.; Dupont, L. Efficient separation of Cobalt, Nickel and Copper using analogues of glycine-betaine based ionic liquids. Sep. Purif. Technol. 2020, 244, 116782. [Google Scholar] [CrossRef]
- Bonhôte, P.; Dias, A.-P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef]
- Gras, M.; Papaiconomou, N.; Chainet, E.; Billard, I. Dicyanamide ions as complexing agents of Co(II): From weak ligands in water to strong ones in an ionic liquid. Solv. Extr. Ion Exch. 2018, 36, 583–601. [Google Scholar] [CrossRef]
- Kobrak, M.; Yager, K. X-ray scattering and physicochemical studies of trialkylamine/carboxylic acid mixtures: Nanoscale structure in pseudoprotic ionic liquids and related solutions. Phys. Chem. Chem. Phys. 2018, 20, 18639–18646. [Google Scholar] [CrossRef]












| Media | Water | Na2SO4 (1.5M) | NaCl 2M | NaCl 4M |
|---|---|---|---|---|
| IL | 19 | 6.9 | 5 | n.m |
| Il/MIBK | 20 | 2.8 | 6 | n.m |
| Il/butyl acetate | 13.5 | 2.4 | 5 | n.m |
| IL/met-THF | 14.75 | 2.75 | 5.9 | n.m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulafrouh, L.; Boudesocque, S.; Mohamadou, A.; Dupont, L. Separation of Co(II) and Ni(II) Using an Analog of Glycine-Betaine Based on Task-Specific Ionic Liquids. Separations 2023, 10, 305. https://doi.org/10.3390/separations10050305
Boulafrouh L, Boudesocque S, Mohamadou A, Dupont L. Separation of Co(II) and Ni(II) Using an Analog of Glycine-Betaine Based on Task-Specific Ionic Liquids. Separations. 2023; 10(5):305. https://doi.org/10.3390/separations10050305
Chicago/Turabian StyleBoulafrouh, Lamia, Stéphanie Boudesocque, Aminou Mohamadou, and Laurent Dupont. 2023. "Separation of Co(II) and Ni(II) Using an Analog of Glycine-Betaine Based on Task-Specific Ionic Liquids" Separations 10, no. 5: 305. https://doi.org/10.3390/separations10050305
APA StyleBoulafrouh, L., Boudesocque, S., Mohamadou, A., & Dupont, L. (2023). Separation of Co(II) and Ni(II) Using an Analog of Glycine-Betaine Based on Task-Specific Ionic Liquids. Separations, 10(5), 305. https://doi.org/10.3390/separations10050305