Preconcentration of Volatile Organic Compounds on Carbon Magnetic Sorbents in the Analysis of Air by Using the Configuration Change of the Sorbent Bed during the Transition from Sorption to Thermodesorption GC-FID
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Adsorbents
2.2. Processing Model Gas Mixtures
2.3. Evaluation of the Adsorption Efficacy
2.4. Instrumentation
3. Results and Discussion
3.1. Morphology of Synthesized Sorbents
3.2. Sorption, Magnetic, and Other Properties of the Studied Sorbents
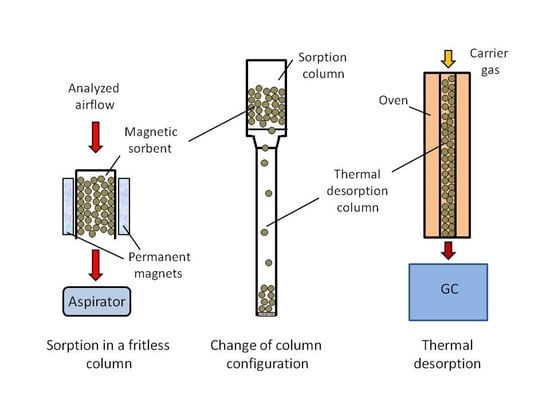
3.3. Preconcentration by Using the Configuration Change of the Magnetic Sorbent Bed
3.4. Advantages of the Proposed Preconcentration Scheme
3.5. Thermal Desorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, H.; Upadhyay, S.; Verma, S. Nanomaterials for air pollution control: A review. IJCRT 2022, 10, 2320–2882. [Google Scholar]
- Mauri-Aucejo, A.R.; Ponce-Catale, P.; Belenguer-Sapina, C. Determination of phenolic compounds in air by using cyclodextrin silica hybrid microporous composite samplers. Talanta 2015, 134, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.; Mester, Z.; Miró, M.; Pedersen-Bjergaard, S.; Pawliszyn, J. Extraction for analytical scale sample preparation (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 649–687. [Google Scholar] [CrossRef] [Green Version]
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: Classification, preparation, and application (with emphasis on aqueous media). Chem. Rev. 2013, 113, 7728–7768. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Ahmadi, F.; Pawliszyn, J. A critical review of solid phase microextraction for analysis of water samples. Trends Anal. Chem. 2016, 85, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Siyuan, D.; Tao, N.; Jing, Y.; Pin, C.; Hao, Y.; Jiahao, W.; Hucheng, Y.; Shukui, Z. Recent advances and applications of magnetic nanomaterials in environmental sample analysis. Trends Anal. Chem. 2020, 126, 115864. [Google Scholar]
- Diwan, A.; Singh, B.; Roychowdhury, T.; Yan, D.D.; Tedone, L.; Nesterenko, P.N.; Paull, B.; Sevy, E.T.; Shellie, R.A.; Kaykhaii, M.; et al. Porous, High Capacity Coatings for Solid Phase Microextraction by Sputtering. Anal. Chem. 2016, 88, 1593–1600. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Rodinkov, O.V.; Pisarev, A.Y.; Moskvin, L.N.; Bugaichenko, A.S.; Nesterenko, P.N. Sensitivity Increase in Headspace Analysis of Hydrocarbons in Water by Using Online Selective Elimination of Gas Extractant. Separations 2022, 9, 15. [Google Scholar] [CrossRef]
- Rodinkov, O.V.; Postnov, V.N.; Bugaychenko, A.S.; Valery, S. Comparison of the capabilities of carbon sorption-active materials for the express concentration of volatile organic compounds from the flow of analysed air. Sorbtsionnye I Khromatograficheskie Protsessy 2021, 21, 307–316. [Google Scholar]
- Maciel, E.V.S.; de Toffoli, A.L.; Neto, E.S.; Nazario, C.E.D.; Lanças, F.M. Trends in analytical chemistry new materials in sample preparation: Recent advances and future trends. Trends Anal. Chem. 2019, 119, 115633. [Google Scholar] [CrossRef]
- Postnov, V.N.; Rodinkov, O.V.; Moskvin, L.N.; Novikov, A.G.; Bugaichenko, A.S.; Krohina, O.A. From carbon nanostructures to high-performance sorbents for chromatographic separation and preconcentration. Russ. Chem. Rev. 2016, 85, 115–138. [Google Scholar] [CrossRef]
- Lanin, S.N.; Rychkova, S.A.; Vinogradov, A.E.; Lanina, K.S.; Obrezkov, O.N.; Nesterenko, P.N. Modification of the surface chemistry of microdispersed sintered detonation nanodiamonds and its effect on the adsorption properties. Adsorpt. J. Int. Adsorpt. Soc. 2017, 23, 639–650. [Google Scholar] [CrossRef]
- Li, W.-K.; Shi, Y.-P. Recent advances and applications of carbon nanotubes based composites in magnetic solid phase extraction. Trends Anal. Chem. 2019, 118, 652. [Google Scholar] [CrossRef]
- Abdelmonaim, A.; Kailasa, S.K.; Lee, S.S.; Rascón, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.-H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. Trends Anal. Chem. 2018, 108, 347. [Google Scholar]
- Koreshkova, A.N.; Gupta, V.; Peristyy, A.; Hasan, C.; Nesterenko, P.; Paull, B. Recent Advances and Applications of Synthetic Diamonds in Solid-Phase Extraction and High-Performance Liquid Chromatography. J. Chromatogr. A 2021, 1640, 461936. [Google Scholar] [CrossRef]
- Nesterenko, P.N.; Fedyanina, O.N. Properties of microdispersed sintered nanodiamonds as a stationary phase for normal-phase high performance liquid chromatography. J. Chromatogr. A 2010, 1217, 498–505. [Google Scholar] [CrossRef]
- Rodinkov, O.V.; Bugaichenko, A.S.; Vlasov, A.Y. Compositional surface-layered sorbents for pre-concentration of organic substances in the air analysis. Talanta 2014, 119, 407–411. [Google Scholar] [CrossRef]
- Rodinkov, O.V.; Vagner, E.A.; Bugaichenko, A.S.; Moskvin, L.N. Comparison of the Efficiencies of Carbon Sorbents for the Preconcentration of Highly Volatile Organic Substances from Wet Gas Atmospheres for the Subsequent Gas-Chromatographic Determination. J. Anal. Chem. 2019, 74, 877–882. [Google Scholar] [CrossRef]
- Postnov, V.N.; Rodinkov, O.V.; Kildiyarova, L.I.; Krokhina, O.A.; Yuriev, G.O.; Murin, I.V. Silica-based composite sorbents and multilayer carbon nanotubes. Russ. J. Gen. Chem. 2022, 92, 323–328. [Google Scholar] [CrossRef]
- Rodinkov, O.; Postnov, V.; Spivakovskyi, V.; Vlasov, A.; Bugaichenko, A.; Slastina, S.; Znamenskaya, E.; Shilov, R.; Lanin, S.; Nesterenko, P. Comparison of adsorbents containing carbon nanotubes for express pre-concentration of volatile organic compounds from the air flow. Separations 2021, 8, 50. [Google Scholar] [CrossRef]
- Rodinkov, O.V.; Moskvin, L.N. Surface-layer composite sorbents for the rapid preconcentration of volatile organic substances from aqueous solutions and gas atmospheres. J. Anal. Chem. 2012, 67, 814–822. [Google Scholar] [CrossRef]
- Nesterenko, E.P.; Nesterenko, P.N.; Connolly, D.; He, X.; Floris, P.; Duffy, E.; Paull, B. Nano-particle modified stationary phases for high-performance liquid chromatography. Analyst 2013, 138, 4229–4254. [Google Scholar] [CrossRef] [PubMed]
- Peristyy, A.A.; Fedyanina, O.N.; Paull, B.; Nesterenko, P.N. Diamond based adsorbents and their application in chromatography. J. Chromatogr. A 2014, 1357, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Rodinkov, O.V.; Bugaichenko, A.S.; Spivakovskyi, V.; Postnov, V.N. Sorption pre-concentration of volatile organic compounds in air analysis with a change in the configuration of the sorption layer in a transition from sorption to thermal desorption. J. Anal. Chem. 2021, 76, 707–713. [Google Scholar] [CrossRef]
- UI-Islam, M.; Ullah, M.W.; Khan, S.; Manan, S.; Khattak, W.A.; Ahmad, W.; Shah, N.; Park, J.K. Current advances of magnetic nanoparticles and degradation of organic pollutants. Environ. Sci. Pollut. Res. 2017, 24, 12713–12722. [Google Scholar] [CrossRef]
- Yu, M.; Wang, L.; Hu, L.; Yaping, L.; Luo, D.; Mei, S. Recent applications of magnetic composites as extraction adsorbents for determination of environmental pollutants. Trends Anal. Chem. 2019, 119, 115611. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, T.; Hao, L.; Guo, Y.; Liu, W.; Guo, L.; Wang, C.; Wang, Z.; Wu, Q. Advances in magnetic porous organic frameworks for analysis and adsorption applications. TrAC Trends Anal. Chem. 2020, 132, 116048. [Google Scholar] [CrossRef]
- Sargazi, M.; Kaykhaii, M. Magnetic Covalent Organic Frameworks—Fundamentals and Applications in Analytical Chemistry. Crit. Rev. Ana. Chem. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Dziubla, T.D.; Hilt, J.Z. Recent advances on iron oxide magnetic nanoparticles as sorbents of organic pollutants in water and wastewater treatment. Rev. Environ. Health 2017, 32, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Tolmacheva, V.V.; Apyari, V.V.; Kochuk, E.V.; Dmitrienko, S.G. Magnetic adsorbents based on iron oxide nanoparticles for the extraction and preconcentration of organic compounds. J. Anal. Chem. 2016, 71, 321–338. [Google Scholar] [CrossRef]
- Lee, P.-L.; Chiu, Y.-K.; Sun, Y.-C.; Ling, Y.-C. Synthesis of a hybrid material consisting of magnetic ironoxide nanoparticles and carbon nanotubes as a gas adsorbent. Carbon 2010, 48, 1397–1404. [Google Scholar] [CrossRef]
- Timofeeva, I.; Alikina, M.; Osmolowsky, M.; Osmolowskaya, O.; Bulatov, A. Magnetic headspace adsorptive microextraction using Fe3O4@Cr(OH)3 nanoparticles for effective determination of volatile phenols. New J. Chem. 2020, 44, 8778–8783. [Google Scholar] [CrossRef]
- Korolev, D.V. Justification of the use of magnetic nanoparticles for targeted drug delivery to ischemic skeletal muscle. Biotechnosphere 2012, 1, 2–6. [Google Scholar]
- ISO 15901-3:2007; Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption—Part 3: Analysis of Micropores by Gas Adsorption. ISO: Geneva, Switzerland, 2007. Available online: http://www.iso.org/standard/40364.html (accessed on 28 February 2023).
- Klyachko-Gurvich, A.L. An improved method of determining surface area by the adsorption of air. Russ. Chem. Bull. 1961, 10, 756–758. [Google Scholar] [CrossRef]
- Platonov, I.A.; Rodinkov, O.V.; Gorbacheva, A.R.; Moskvin, L.N.; Kolesnichenko, I.N. Methods and devices for the preparation of standard gas mixtures. J. Anal. Chem. 2018, 73, 109–127. [Google Scholar] [CrossRef]
- Vitenberg, A.G.; Konopel’ko, L.A. Gas chromatographic headspace analysis: Metrological aspects. J. Anal. Chem. 2011, 66, 438–457. [Google Scholar] [CrossRef]
- Nemirovskiy, A.M. Calculations in frontal chromatography. Ind. Lab. 1996, 62, 13–18. [Google Scholar]
- Satterfield, C.N. Heterogeneous Catalysis in Practice; Mc-Hill Publ: New York, NY, USA, 1980; 416p. [Google Scholar]
- Melekhin, A.O.; Tolmacheva, V.V.; Shubina, E.G.; Dmitrienko, S.G.; Apyari, V.V.; Grudev, A.I. Determination of nitrofuran metabolites in honey using a new derivatization reagent, magnetic solid-phase extraction and LC–MS/MS. Talanta 2021, 230, 122310. [Google Scholar] [CrossRef]
- Tsysin, G.I.; Kovalev, I.A.; Nesterenko, P.N.; Penner, N.A.; Filippov, O.A. Application of linear model of sorption dynamics to the comparison of solid phase extraction systems of phenol. Sep. Purif. Technol. 2003, 33, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Gritti, F.; Guiochon, G. The van Deemter equation: Assumptions, limits, and adjustment to modern high performance liquid chromatography. J. Chromatiogr. A 2013, 1302, 1–13. [Google Scholar] [CrossRef]
- Tsizin, G.I. Concerning the Concentration Factor. J. Anal. Chem. 2011, 11, 535. [Google Scholar] [CrossRef] [Green Version]
- Loevkvist, P.; Joensson, A. Capacity of sampling and pre-concentration columns with a low number of theoretical plates. Anal. Chem. 1987, 59, 818–821. [Google Scholar] [CrossRef]
- Tsizin, G.I.; Statkus, M.A. Sorbtsionnoe Kontsentrirovanie Mikrokomponentov v Dinamicheskikh Usloviyakh (Adsorption Preconcentration of Trace Components under Dynamic Conditions); LENAND: Moscow, Russia, 2016; p. 480. [Google Scholar]
- ISO 16017-1:2000; Indoor, Ambient and Workplace Air—Sampling and Analysis of Volatile Organic Compounds by Sorbents Tube/Thermal Desorption/Capillary Gas Chromatography—Part 1: Pumped Sampling Annex F. ISO: Geneva, Switzerland, 2002.
- Zhang, S.; Yao, W.; Ying, J.; Zhao, H. Polydopamine-reinforced magnetization of zeolitic imidazolate framework ZIF-7 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from the air-water environment. J. Chromatogr. A 2016, 1452, 18–26. [Google Scholar] [CrossRef] [PubMed]








| Sorption Tube Number | r, cm | S, cm2 | l, cm | V, cm3 |
|---|---|---|---|---|
| 1 | 0.13 | 0.053 | 10.2 | 0.54 |
| 2 | 0.18 | 0.102 | 5.4 | 0.55 |
| 3 | 0.33 | 0.342 | 1.6 | 0.55 |
| 4 | 0.45 | 0.636 | 0.88 | 0.56 |
| Adsorbent | dp, μm | Ssp, m2/g | Carbon Content, % | Hydrophilicity, % | Is, emf/g | Specific Retention Volume, dm3/g | |
|---|---|---|---|---|---|---|---|
| Butanol-1 | Phenol | ||||||
| I | 50–100 | 20 | 1.3 | 1.7 | 173 | 43 | 230 |
| II | 100–150 | 26 | 6.2 | 1.4 | 68 | 56 | 420 |
| III | 180–250 | 9.6 | 1.4 | <0.1 | 41 | 1.7 | 8.2 |
| IV | 180–250 | 17 | 3.3 | <0.1 | 55 | 3.1 | 14 |
| Carbopack C | 180–250 | 12 | >98 | 0.3 | - | 4.3 | 29 |
| Carbopack B | 180–250 | 110 | >98 | 1.3 | - | 28 | 340 |
| Carbopack X | 180–250 | 240 | >98 | 1.5 | - | 69 | 730 |
| Component, Sorbent | Column (Number, Size) | WG, mL/min | uG, cm/s | VB, L | Kcon | t, min | E, min−1 |
|---|---|---|---|---|---|---|---|
| Butanol-1, adsorbent II | 1; (10.2 × 0.26) cm | 50 | 20.9 | 12.0 | 2.2 × 104 | 240 | 91.6 |
| 2; (5.4 × 0.36) cm | 100 | 21.7 | 11.3 | 2.1 × 104 | 112 | 188 | |
| 3; (1.6 × 0.66) cm | 300 | 19.0 | 10.6 | 1.9 × 104 | 35 | 542 | |
| 4; (0.88 × 0.9) cm | 500 | 17.4 | 7.7 | 1.4 × 104 | 15.4 | 909 | |
| Phenol, adsorbent IV | 1; (10.2 × 0.26) cm | 50 | 20.9 | 1.92 | 3.5 × 103 | 38.4 | 90.6 |
| 2; (5.4 × 0.36) cm | 100 | 21.7 | 2.01 | 3.6 × 103 | 20.1. | 179 | |
| 3; (1.6 × 0.66) cm | 300 | 19.0 | 1.81 | 3.2 × 103 | 6.0 | 533 | |
| 4; (0.88 × 0.9) cm | 500 | 17.4 | 1.67 | 3.0 × 103 | 3.34 | 898 |
| Analyte | Sorbent | Concentration Time, min | Flow Rate, mL/min | Determination Results μg/m3 | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | ||
| Butanol-1 | Adsorbent II | 20 | 2 | 100 | 1000 | 96 ± 6 | 91 ± 7 |
| Pentanol-1 | 98 ± 5 | 94 ± 6 | |||||
| Phenol | Adsorbent IV | 30 | 3 | 70 | 700 | 89 ± 10 | 83 ± 11 |
| o-Cresol | 94 ± 8 | 95 ± 9 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodinkov, O.; Postnov, V.; Spivakovskyi, V.; Znamenskaya, E.; Zheludovskaya, A.; Nesterenko, P. Preconcentration of Volatile Organic Compounds on Carbon Magnetic Sorbents in the Analysis of Air by Using the Configuration Change of the Sorbent Bed during the Transition from Sorption to Thermodesorption GC-FID. Separations 2023, 10, 416. https://doi.org/10.3390/separations10070416
Rodinkov O, Postnov V, Spivakovskyi V, Znamenskaya E, Zheludovskaya A, Nesterenko P. Preconcentration of Volatile Organic Compounds on Carbon Magnetic Sorbents in the Analysis of Air by Using the Configuration Change of the Sorbent Bed during the Transition from Sorption to Thermodesorption GC-FID. Separations. 2023; 10(7):416. https://doi.org/10.3390/separations10070416
Chicago/Turabian StyleRodinkov, Oleg, Victor Postnov, Valery Spivakovskyi, Ekaterina Znamenskaya, Anastasia Zheludovskaya, and Pavel Nesterenko. 2023. "Preconcentration of Volatile Organic Compounds on Carbon Magnetic Sorbents in the Analysis of Air by Using the Configuration Change of the Sorbent Bed during the Transition from Sorption to Thermodesorption GC-FID" Separations 10, no. 7: 416. https://doi.org/10.3390/separations10070416
APA StyleRodinkov, O., Postnov, V., Spivakovskyi, V., Znamenskaya, E., Zheludovskaya, A., & Nesterenko, P. (2023). Preconcentration of Volatile Organic Compounds on Carbon Magnetic Sorbents in the Analysis of Air by Using the Configuration Change of the Sorbent Bed during the Transition from Sorption to Thermodesorption GC-FID. Separations, 10(7), 416. https://doi.org/10.3390/separations10070416