Enantioselective Separation of Synthetic Cathinones by Capillary Electrophoresis with Ionic Liquid and Cyclodextrin Buffer Co-Additives
Abstract
:1. Introduction
2. Materials and Methods
3. Results & Discussion
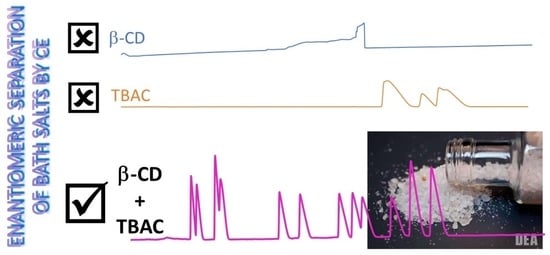
3.1. Enantiomeric Separations of Synthetic Cathinones by Capillary Electrophoresis
3.2. TBAC Concentration Study
3.3. Effects of Background Electrolyte, Capillary Dimensions, Temperature, and Separation Voltage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schmitt-Kopplin, P.; Fekete, A. The CE-Way of Thinking: “All Is Relative!”. Methods Mol. Biol. 2016, 1483, 3–19. [Google Scholar] [CrossRef]
- Voeten, R.L.C.; Ventouri, I.K.; Haselberg, R.; Somsen, G.W. Capillary Electrophoresis: Trends and Recent Advances. Anal. Chem. 2018, 90, 1464–1481. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, A.J.; Armistead, P.M.; Allbritton, N.L. Automated Capillary Electrophoresis System for Fast Single-Cell Analysis. Anal. Chem. 2013, 85, 4797–4804. [Google Scholar] [CrossRef] [Green Version]
- Rabel, S.R.; Stobaugh, J.F. Applications of Capillary Electrophoresis in Pharmaceutical Analysis. Pharm. Res. 1993, 10, 171–186. [Google Scholar] [CrossRef]
- Sniehotta, M.; Schiffer, E.; Zürbig, P.; Novak, J.; Mischak, H. CE—A Multifunctional Application for Clinical Diagnosis. Electrophoresis 2007, 28, 1407–1417. [Google Scholar] [CrossRef]
- Krait, S.; Konjaria, M.L.; Scriba, G.K.E. Advances of Capillary Electrophoresis Enantioseparations in Pharmaceutical Analysis (2017–2020). Electrophoresis 2021, 42, 1709–1725. [Google Scholar] [CrossRef]
- Chankvetadze, B. Application of Enantioselective Separation Techniques to Bioanalysis of Chiral Drugs and Their Metabolites. TrAC Trends Anal. Chem. 2021, 143, 116332. [Google Scholar] [CrossRef]
- Weinberger, R.; Lurie, I.S. Micellar Electrokinetic Capillary Chromatography of Illicit Drug Substances. Anal. Chem. 1991, 63, 823–827. [Google Scholar] [CrossRef]
- Wernly, P.; Thormann, W. Analysis of Illicit Drugs in Human Urine by Micellar Electrokinetic Capillary Chromatography with On-Column Fast Scanning Polychrome Absorption Detection. Anal. Chem. 1991, 63, 2878–2882. [Google Scholar] [CrossRef]
- Nowak, P.M.; Olesek, K.; Woźniakiewicz, M.; Mitoraj, M.; Sagan, F.; Kościelniak, P. Cyclodextrin-Induced Acidity Modification of Substituted Cathinones Studied by Capillary Electrophoresis Supported by Density Functional Theory Calculations. J. Chromatogr. A 2018, 1580, 142–151. [Google Scholar] [CrossRef]
- Baciu, T.; Borrull, F.; Calull, M.; Aguilar, C. Enantioselective Determination of Cathinone Derivatives in Human Hair by Capillary Electrophoresis Combined In-Line with Solid-Phase Extraction. Electrophoresis 2016, 37, 2352–2362. [Google Scholar] [CrossRef]
- German, C.L.; Fleckenstein, A.E.; Hanson, G.R. Bath Salts and Synthetic Cathinones: An Emerging Designer Drug Phenomenon. Life Sci. 2014, 97, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geresu, B. Khat (Catha edulis F.) and Cannabinoids: Parallel and Contrasting Behavioral Effects in Preclinical and Clinical Studies. Pharmacol. Biochem. Behav. 2015, 138, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Costa, V.M.; de Lourdes Bastos, M.; Carvalho, F.; Capela, J.P. An Updated Review on Synthetic Cathinones. Arch. Toxicol. 2021, 95, 2895–2940. [Google Scholar] [CrossRef]
- Almeida, A.S.; Silva, B.; de Pinho, P.G.; Remião, F.; Fernandes, C. Synthetic Cathinones: Recent Developments, Enantioselectivity Studies and Enantioseparation Methods. Molecules 2022, 27, 2057. [Google Scholar] [CrossRef]
- Saz, J.M.; Marina, M.L. Recent Advances on the Use of Cyclodextrins in the Chiral Analysis of Drugs by Capillary Electrophoresis. J. Chromatogr. A 2016, 1467, 79–94. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. Native and Substituted Cyclodextrins as Chiral Selectors for Capillary Electrophoresis Enantioseparations: Structures, Features, Application, and Molecular Modeling. Electrophoresis 2021, 42, 1676–1708. [Google Scholar] [CrossRef]
- Zhu, Q.; Scriba, G.K.E. Advances in the Use of Cyclodextrins as Chiral Selectors in Capillary Electrokinetic Chromatography: Fundamentals and Applications. Chromatographia 2016, 79, 1403–1435. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to Explore the Mechanism to Form Inclusion Complexes of β-Cyclodextrin with Vitamin Molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chankvetadze, B.; Scriba, G.K.E. Cyclodextrins as Chiral Selectors in Capillary Electrophoresis: Recent Trends in Mechanistic Studies. TrAC Trends Anal. Chem. 2023, 160, 116987. [Google Scholar] [CrossRef]
- Pérez-Alcaraz, A.; Borrull, F.; Aguilar, C.; Calull, M. An Electrokinetic Supercharging Approach for the Enantiodetermination of Cathinones in Urine Samples by Capillary Electrophoresis. Microchem. J. 2020, 158, 105300. [Google Scholar] [CrossRef]
- Kowalski, P.; Olędzka, I.; Plenis, A.; Roszkowska, A.; Bączek, T. Strengths and Weaknesses of Ionic Liquids as Efficiency Enhancers in Capillary Electrophoresis. TrAC Trends Anal. Chem. 2023, 162, 117031. [Google Scholar] [CrossRef]
- Wahl, J. The Use of Ionic Liquids in Capillary Electrophoresis Enantioseparation; Universität Würzburg: Würzburg, Germany, 2019. [Google Scholar]
- Pham, T.P.T.; Cho, C.-W.; Yun, Y.-S. Environmental Fate and Toxicity of Ionic Liquids: A Review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Modification of Electroosmotic Flow with Cetyltrimethylammonium Bromide in Capillary Zone Electrophoresis. J. High Res. Chrom. 1987, 10, 622–624. [Google Scholar] [CrossRef]
- Huang, X.; Luckey, J.A.; Gordon, M.J.; Zare, R.N. Quantitative Determination of Low Molecular Weight Carboxylic Acids by Capillary Zone Electrophoresis/Conductivity Detection. Anal. Chem. 1989, 61, 766–770. [Google Scholar] [CrossRef]
- Greño, M.; Marina, M.L.; Castro-Puyana, M. Enantioseparation by Capillary Electrophoresis Using Ionic Liquids as Chiral Selectors. Crit. Rev. Anal. Chem. 2018, 48, 429–446. [Google Scholar] [CrossRef]
- Zhang, Q. Ionic Liquids in Capillary Electrophoresis for Enantioseparation. TrAC Trends Anal. Chem. 2018, 100, 145–154. [Google Scholar] [CrossRef]
- Hui, B.Y.; Raoov, M.; Zain, N.N.M.; Mohamad, S.; Osman, H. Combination of Cyclodextrin and Ionic Liquid in Analytical Chemistry: Current and Future Perspectives. Crit. Rev. Anal. Chem. 2017, 47, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, J.; Bianchi, V.; Shamsi, S.A. Combined Use of Chiral Ionic Liquid and CD for MEKC: Part II Determination of Binding Constants. Electrophoresis 2009, 30, 2820–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzgrabe, U.; Wahl, J. Ionic Liquids in Capillary Electrophoresis. In Capillary Electrophoresis. Methods in Molecular Biology; Schmitt-Kopplin, P., Ed.; Humana Press: New York, NY, USA, 2016; Volume 1483. [Google Scholar] [CrossRef]
- Wahl, J.; Holzgrabe, U. Capillary Electrophoresis Separation of Phenethylamine Enantiomers Using Amino Acid Based Ionic Liquids. J. Pharm. Biomed. Anal. 2018, 148, 245–250. [Google Scholar] [CrossRef]
- Schmid, M.G.; Hägele, J.S. Separation of Enantiomers and Positional Isomers of Novel Psychoactive Substances in Solid Samples by Chromatographic and Electrophoretic Techniques—A Selective Review. J. Chromatogr. A 2020, 1624, 461256. [Google Scholar] [CrossRef]
- Wren, S.; Berger, T.A.; Boos, K.-S.; Engelhardt, H.; Adlard, E.R.; Davies, I.W.; Altria, K.D.; Stock, R. The Use of Cyclodextrins as Chiral Selectors. In The Separation of Enantiomers by Capillary Electrophoresis; Chromatographia CE Series; Wren, S., Berger, T.A., Boos, K.-S., Engelhardt, H., Adlard, E.R., Davies, I.W., Altria, K.D., Stock, R., Eds.; Vieweg + Teubner Verlag: Wiesbaden, Germany, 2001; Volume 6, pp. 59–77. [Google Scholar] [CrossRef]
- Dougherty, A.M.; Cooke, N.; Shieh, P. Capillary Surface Modification in Capillary Electrophoresis. In Handbook of Capillary Electrophoresis, 2nd ed.; Landers, J.P., Ed.; CRC Press: Boca Raton, FL, USA, 1997; Chapter 24; pp. 675–715. [Google Scholar]
- Dolnik, V. Borate-Containing Background Electrolytes to Improve CE Separation in Bare Capillaries. Electrophoresis 2020, 41, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Bean, S.R.; Lookhart, G.L.; Bietz, J.A. Acetonitrile as a Buffer Additive for Free Zone Capillary Electrophoresis Separation and Characterization of Maize (Zea mays L.) and Sorghum (Sorghum bicolor L. Moench) Storage Proteins. J. Agric. Food Chem. 2000, 48, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Viskari, P.J.; Colyer, C.L. Separation and Quantitation of Phycobiliproteins Using Phytic Acid in Capillary Electrophoresis with Laser-Induced Fluorescence Detection. J. Chromatogr. A 2002, 972, 269–276. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, J.B.; Colyer, C.L. Enantioselective Separation of Synthetic Cathinones by Capillary Electrophoresis with Ionic Liquid and Cyclodextrin Buffer Co-Additives. Separations 2023, 10, 417. https://doi.org/10.3390/separations10070417
Roberts JB, Colyer CL. Enantioselective Separation of Synthetic Cathinones by Capillary Electrophoresis with Ionic Liquid and Cyclodextrin Buffer Co-Additives. Separations. 2023; 10(7):417. https://doi.org/10.3390/separations10070417
Chicago/Turabian StyleRoberts, Jennifer Buchanan, and Christa L. Colyer. 2023. "Enantioselective Separation of Synthetic Cathinones by Capillary Electrophoresis with Ionic Liquid and Cyclodextrin Buffer Co-Additives" Separations 10, no. 7: 417. https://doi.org/10.3390/separations10070417
APA StyleRoberts, J. B., & Colyer, C. L. (2023). Enantioselective Separation of Synthetic Cathinones by Capillary Electrophoresis with Ionic Liquid and Cyclodextrin Buffer Co-Additives. Separations, 10(7), 417. https://doi.org/10.3390/separations10070417