Enhanced CO2 Capture by Sorption on Electrospun Poly (Methyl Methacrylate)
Abstract
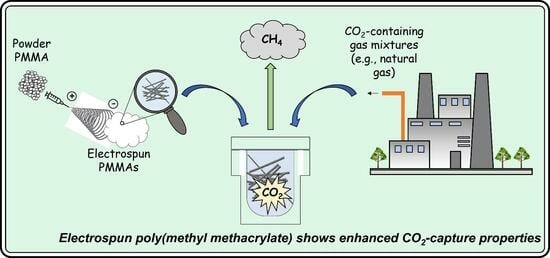
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Experimental Apparatus
2.3. Determination of CO2 Capture
2.4. Method Validation
2.5. Brunauer–Emmett–Teller (BET) Analysis
2.6. Scanning Electron Microscopy
2.7. AFM Analysis
2.8. TGA Analysis
2.9. FT-IR Analysis
3. Results
3.1. Sorption Experiments
- a.
- Powder PMMA
- b.
- Positive electrospun PMMA (ePMMA+)
- c.
- Negative electrospun PMMA (ePMMA-)
3.2. Structural Characterization of Electrospun PMMAs
- a.
- AFM
- b.
- SEM
- c.
- TGA
- d.
- BET
- e.
- FT-IR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nemitallah, M.A.; Habib, M.A.; Badr, H.M.; Said, S.A.; Jamal, A.; Ben-Mansour, R.; Mokheimer, E.M.A.; Mezghani, K. Oxy-fuel combustion technology: Current status, applications, and trends. Int. J. Energy Res. 2017, 41, 1670–1708. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Aghel, B.; Janati, S.; Wongwises, S.; Shadloo, M.S. Review on CO2 capture by blended amine solutions. Int. J. Greenh. Gas Control 2022, 119, 103715. [Google Scholar] [CrossRef]
- Yu, C.; Jia, Y.; Fang, K.; Qin, Y.; Deng, N.; Liang, Y. Preparation hierarchical porous MOF membranes with island-like structure for efficient gas separation. J. Memb. Sci. 2022, 663, 121036. [Google Scholar] [CrossRef]
- Kadirkhan, F.; Goh, P.S.; Ismail, A.F.; Wan Mustapa, W.N.F.; Halim, M.H.M.; Soh, W.K.; Yeo, S.Y. Recent Advances of Polymeric Membranes in Tackling Plasticization and Aging for Practical Industrial CO2/CH4 Applications—A Review. Membranes 2022, 12, 71. [Google Scholar] [CrossRef]
- Wissinger, R.G.; Paulaitis, M.E. Swelling and sorption in polymer–CO2 mixtures at elevated pressures. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 2497–2510. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.H.; Ryu, Y.; Cha, S.W. Modeling and Experiment for the Diffusion Coefficient of Subcritical Carbon Dioxide in Poly(methyl methacrylate) to Predict Gas Sorption and Desorption. Polymers 2022, 14, 596. [Google Scholar] [CrossRef]
- Wissinger, R.G.; Paulaitis, M.E. Molecular Thermodynamic Model for Sorption and Swelling in Glassy Polymer-CO2 Systems at Elevated Pressures. Ind. Eng. Chem. Res. 1991, 30, 842–851. [Google Scholar] [CrossRef]
- Aubert, J.H. Solubility of carbon dioxide in polymers by the quartz crystal microbalance technique. J. Supercrit. Fluids 1998, 11, 163–172. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Noon, M.S.; Tomasko, D.L. CO2-induced PMMA swelling and multiple thermodynamic property analysis using Sanchez-Lacombe EOS. Macromolecules 2005, 38, 4416–4424. [Google Scholar] [CrossRef]
- Webb, K.F.; Teja, A.S. Solubility and diffusion of carbon dioxide in polymers. Fluid Phase Equilib. 1999, 158–160, 1029–1034. [Google Scholar] [CrossRef]
- Kiran, E.; Sarver, J.A.; Hassler, J.C. Solubility and diffusivity of CO2 and N2 in polymers and polymer swelling, glass transition, melting, and crystallization at high pressure: A critical review and perspectives on experimental methods, data, and modeling. J. Supercrit. Fluids 2022, 185, 105378. [Google Scholar] [CrossRef]
- Vopička, O.; De Angelis, M.G.; Du, N.; Li, N.; Guiver, M.D.; Sarti, G.C. Mixed gas sorption in glassy polymeric membranes: II. CO2/CH4 mixtures in a polymer of intrinsic microporosity (PIM-1). J. Memb. Sci. 2014, 459, 264–276. [Google Scholar] [CrossRef]
- Notario, B.; Pinto, J.; Rodríguez-Pérez, M.A. Towards a new generation of polymeric foams: PMMA nanocellular foams with enhanced physical properties. Polymers 2015, 63, 116–126. [Google Scholar] [CrossRef]
- Shi, Z.; Ma, X.; Zhao, G.; Wang, G.; Zhang, L.; Li, B. Fabrication of high porosity Nanocellular polymer foams based on PMMA/PVDF blends. Mater. Des. 2020, 195, 109002. [Google Scholar] [CrossRef]
- Kwon, Y.K.; Bae, H.K. Production of microcellular foam plastics by supercritical carbon dioxide. Korean J. Chem. Eng. 2007, 24, 127–132. [Google Scholar] [CrossRef]
- Koros, W.J.; Smith, G.N.; Stannett, V. High-pressure sorption of carbon dioxide in solvent-cast poly(methyl methacrylate) and poly(ethyl methacrylate) films. J. Appl. Polym. Sci. 1981, 26, 159–170. [Google Scholar] [CrossRef]
- Fried, J.R.; Li, W. High-pressure FTIR studies of gas–polymer interactions. J. Appl. Polym. Sci. 1990, 41, 1123–1131. [Google Scholar] [CrossRef]
- Edwards, R.R.; Tao, Y.; Xu, S.; Wells, P.S.; Yun, K.S.; Parcher, J.F. Chromatographic investigation of CO2-polymer interactions at near-critical conditions. J. Phys. Chem. B 1998, 102, 1287–1295. [Google Scholar] [CrossRef]
- Shoghl, S.N.; Pazuki, G.; Raisi, A. A model to predict the solubility and permeability of gaseous penetrant in the glassy polymeric membrane at high pressure. J. Appl. Polym. Sci. 2021, 138, 50548. [Google Scholar] [CrossRef]
- Nelson, M.R.; Borkman, R.F. Ab initio calculations on CO2 binding to carbonyl groups. J. Phys. Chem. A 1998, 102, 7860–7863. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Liu, K.H. The effect of carbonyl group on sorption of CO2 in glassy polymers. J. Supercrit. Fluids 2003, 25, 261–268. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamamoto, Y.; Nawaby, A.V.; Handa, Y.P. Raman spectroscopic investigation of the phase behavior and phase transitions in a poly(methyl methacrylate)-carbon dioxide system. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2214–2217. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Liu, K.H. Solubility of CO2 in glassy PMMA and PS over a wide pressure range: The effect of carbonyl groups. J. Polym. Res. 2002, 9, 107–113. [Google Scholar] [CrossRef]
- Ricci, E.; De Angelis, M.G.; Minelli, M. A comprehensive theoretical framework for the sub and supercritical sorption and transport of CO2 in polymers. Chem. Eng. J. 2022, 435, 135013. [Google Scholar] [CrossRef]
- Di Noto, V.; Vezzù, K.; Giffin, G.A.; Conti, F.; Bertucco, A. Effect of high pressure CO2 on the structure of PMMA: A FT-IR study. J. Phys. Chem. B 2011, 115, 13519–13525. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, Y.; Mizoguchi, K.; Terada, K.; Fujiwara, Y.; Wang, J.S. CO2 sorption and dilation of poly(methyl methacrylate). Macromolecules 1998, 31, 472–478. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kawamura, T.; Takeya, S.; Jin, S.; Yamamoto, Y.; Komai, T.; Takahashi, M.; Nawaby, A.V.; Handa, Y.P. Probing fickian and non-fickian diffusion of CO2 in poly(methyl methacrylate) using in situ Raman spectroscopy and microfocus X-ray computed tomography. Macromolecules 2004, 37, 9302–9304. [Google Scholar] [CrossRef]
- Wissinger, R.G.; Paulaitis, M.E. Glass transitions in polymer / CO2 mixtures at elevated pressures. J. Polym. Sci. Part B Polym. Phys. 1991, 29, 631–633. [Google Scholar] [CrossRef]
- Condo, P.D.; Johnston, K.P. In situ measurement of the glass transition temperature of polymers with compressed fluid diluents. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 523–533. [Google Scholar] [CrossRef]
- Baldanza, A.; Loianno, V.; Mensitieri, G.; Scherillo, G. Modelling changes in glass transition temperature in polymer matrices exposed to low molecular weight penetrants. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2023, 381, 20210216. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, D.C.; Carrascal, D.; Solórzano, E.; Pérez, M.A.R.; Pinto, J. Analysis of the retrograde behavior in PMMA-CO2 systems by measuring the (effective) glass transition temperature using refractive index variations. J. Supercrit. Fluids 2021, 170, 105159. [Google Scholar] [CrossRef]
- Handa, Y.P.; Zhang, Z. A new technique for measuring retrograde vitrification in polymer-gas systems and for making ultramicrocellular foams from the retrograde phase. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 716–725. [Google Scholar] [CrossRef]
- Guo, H.; Kumar, V. Solid-state poly(methyl methacrylate) (PMMA) nanofoams. Part I: Low-temperature CO2 sorption, diffusion, and the depression in PMMA glass transition. Polymers 2015, 57, 157–163. [Google Scholar] [CrossRef]
- Condo, P.D.; Sanchez, I.C.; Panayiotou, C.G.; Johnston, K.P. Glass Transition Behavior Including Retrograde Vitrification of Polymers with Compressed Fluid Diluents. Macromolecules 1992, 25, 6119–6127. [Google Scholar] [CrossRef]
- Condo, P.D.; Johnston, K.P. Retrograde Vitrification of Polymers With Compressed Fluid Diluents: Experimental Confirmation. Macromolecules 1992, 25, 6730–6732. [Google Scholar] [CrossRef]
- Mohammadi, M.; Fazli, H.; Karevan, M.; Davoodi, J. The glass transition temperature of PMMA: A molecular dynamics study and comparison of various determination methods. Eur. Polym. J. 2017, 91, 121–133. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Fang, T. Experimental research on swelling and glass transition behavior of poly(methyl methacrylate) in supercritical carbon dioxide. J. Supercrit. Fluids 2016, 110, 110–116. [Google Scholar] [CrossRef]
- Ikeda-Fukazawa, T.; Kita, D.; Nagashima, K. Raman spectroscopic study of CO2 sorption process in poly methyl methacrylate. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 831–842. [Google Scholar] [CrossRef]
- Braeuer, A.S. Prospects: Facing current challenges in high pressure high temperature process engineering with in situ Raman measurements. J. Supercrit. Fluids 2018, 134, 80–87. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Panayiotou, C. Simultaneous determination of sorption, heat of sorption, diffusion coefficient and glass transition depression in polymer-CO2 systems. Thermochim. Acta. 2011, 521, 98–106. [Google Scholar] [CrossRef]
- Eslami, H.; Kesik, M.; Karimi-Varzaneh, H.A.; Müller-Plathe, F. Sorption and diffusion of carbon dioxide and nitrogen in poly(methyl methacrylate). J. Chem. Phys. 2013, 139, 124902. [Google Scholar] [CrossRef]
- Taguchi, T.; Saito, H. Effects of plasticization and hydrostatic pressure on tensile properties of PMMA under compressed carbon dioxide and nitrogen. J. Appl. Polym. Sci. 2016, 133, 43431. [Google Scholar] [CrossRef]
- Tang, Q.; Yang, B.; Zhao, Y.; Zhao, L. Sorption and diffusion of sub/supercritical carbon dioxide in poly(methyl methacrylate). J. Macromol. Sci. Part B Phys. 2007, 46, 275–284. [Google Scholar] [CrossRef]
- Zhou, L.; Dai, X.; Du, J.; Wang, T.; Wu, L.; Tang, Y.; Shen, J. Fabrication of Poly(MMA- co -ST) Hybrid Membranes Containing AgCl Nanoparticles by in Situ Ionic Liquid Microemulsion Polymerization and Enhancement of Their Separation Performance. Ind. Eng. Chem. Res. 2015, 54, 3326–3332. [Google Scholar] [CrossRef]
- Shen, J.-N.; Zheng, X.-C.; Ruan, H.-M.; Wu, L.-G.; Qiu, J.-H.; Gao, C.-J. Synthesis of AgCl/PMMA hybrid membranes and their sorption performance of cyclohexane/cyclohexene. J. Memb. Sci. 2007, 304, 118–124. [Google Scholar] [CrossRef]
- Maroni, F.; Bruni, P.; Suzuki, N.; Aihara, Y.; Croce, F. Electrospun tin-carbon nanocomposite as anode material for all solid state lithium-ion batteries. J. Solid State Electrochem. 2019, 23, 1697–1703. [Google Scholar] [CrossRef]
- Di Profio, P.; Canale, V.; D’Alessandro, N.; Germani, R.; Di Crescenzo, A.; Fontana, A. Separation of CO2 and CH4 from Biogas by Formation of Clathrate Hydrates: Importance of the Driving Force and Kinetic Promoters. ACS Sustain. Chem. Eng. 2017, 5, 1990–1997. [Google Scholar] [CrossRef]
- Canale, V.; Fontana, A.; Siani, G.; Di Profio, P. Hydrate Induction Time with Temperature Steps: A Novel Method for the Determination of Kinetic Parameters. Energy Fuels 2019, 33, 6113–6118. [Google Scholar] [CrossRef]
- Arca, S.; Di Profio, P.; Germani, R.; Savelli, G. Apparatus for Preparing and Studying Clathrate Hydrates. WO2007122647A1, 21 April 2006. [Google Scholar]
- Gazzani, M.; Macchi, E.; Manzolini, G. CO2 capture in natural gas combined cycle with SEWGS. Part A: Thermodynamic performances. Int. J. Greenh. Gas Control 2013, 12, 493–501. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chiou, J.S.; Paul, D.R. Effects of CO2 exposure on gas transport properties of glassy polymers. J. Memb. Sci. 1987, 32, 195–205. [Google Scholar] [CrossRef]
- Miranda, L.D.; Bell, R.J.; Short, R.T.; van Amerom, F.H.W.; Byrne, R.H. The influence of hydrostatic pressure on gas diffusion in polymer and nano-composite membranes: Application to membrane inlet mass spectrometry. J. Memb. Sci. 2011, 385–386, 49–56. [Google Scholar] [CrossRef]
- Neyertz, S.; Brown, D. Molecular dynamics study of carbon dioxide sorption and plasticization at the interface of a glassy polymer membrane. Macromolecules 2013, 46, 2433–2449. [Google Scholar] [CrossRef]
- Hirota, S.I.; Tominaga, Y.; Asai, S.; Sumita, M. Dielectric relaxation behavior of poly(methyl methacrylate) under high-pressure carbon dioxide. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2951–2962. [Google Scholar] [CrossRef]
- Zhang, Z.; Handa, Y.P. An in situ study of plasticization of polymers by high-pressure gases. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 977–982. [Google Scholar] [CrossRef]
- Kwag, C.; Manke, C.W.; Gulari, E. Rheology of molten polystyrene with dissolved supercritical and near-critical gases. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2771–2781. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Rodríguez, J.F.; Gracia, I.; de Lucas, A.; García, M.T. Modification of polystyrene properties by CO2: Experimental study and correlation. J. Appl. Polym. Sci. 2015, 132, 41696. [Google Scholar] [CrossRef]
- Kalospiros, N.S.; Paulaitis, M.E. Molecular thermodynamic model for solvent-induced glass transitions in polymer—Supercritical fluid systems. Chem. Eng. Sci. 1994, 49, 659–668. [Google Scholar] [CrossRef]
- Chiou, J.S.; Barlow, J.W.; Paul, D.R. Plasticization of glassy polymers by CO2. J. Appl. Polym. Sci. 1985, 30, 2633–2642. [Google Scholar] [CrossRef]









| pPMMA | ||||||
|---|---|---|---|---|---|---|
| 20 °C | 1 °C | |||||
| I | II | III | I | II | III | |
| 1 MPa | 8.0 ± 1.8 | 6.1 ± 1.2 | 5.9 ± 1.3 | 5.3 ± 0.9 | 3.8 ± 0.9 | 4.7 ± 1.1 |
| 2 MPa | 45.8 ± 1.9 | 40.1 ± 3.0 | 40.4 ± 2.4 | 59.3 ± 0.8 | 51.8 ± 1.2 | 56.6 ± 2.0 |
| 3 MPa | 114.3 ± 3.2 | 91.2 ± 2.9 | 87.1 ± 2.7 | 206.3 ± 3.8 | 166.2 ± 3.2 | 168.6 ± 3.3 |
| 4 MPa | 158.2 ± 2.5 | 118.0 ± 3.1 | 121.0 ± 3.1 | --- | --- | --- |
| ePMMA+ | ||||||
| 20 °C | 1 °C | |||||
| I | II | III | I | II | III | |
| 1 MPa | 46.1 ± 1.5 | 43.8 ± 1.3 | 43.6 ± 1.8 | 60.6 ± 1.7 | 64.4 ± 1.4 | 64.7 ± 2.0 |
| 2 MPa | 84.2 ± 2.1 | 83.0 ± 2.2 | 81.7 ± 2.8 | 143.3 ± 3.5 | 134.5 ± 3.7 | 138.1 ± 3.3 |
| 3 MPa | 124.9 ± 3.8 | 121.9 ± 3.5 | 118.4 ± 2.2 | 259.2 ± 4.0 | 223.9 ± 4.2 | 227.2 ± 4.6 |
| 4 MPa | 142.2 ± 4.1 | 148.9 ± 3.6 | 143.9 ± 3.1 | --- | --- | --- |
| ePMMA- | ||||||
| 20 °C | 1 °C | |||||
| I | II | III | I | II | III | |
| 1 MPa | 46.0 ± 1.0 | 45.9 ± 2.1 | 45.3 ± 1.6 | 56.8 ± 2.1 | 55.4 ± 1.4 | 57.0 ± 1.1 |
| 2 MPa | 96.1. ± 2 | 91.5 ± 2.2 | 85.8 ± 1.9 | 141.4 ± 3.0 | 139.5 ± 2.3 | 134.0 ± 2.8 |
| 3 MPa | 126.0 ± 2.5 | 114.7 ± 3.8 | 114.4 ± 2.1 | 201.7 ± 4.2 | 195.0 ± 4.5 | 201.9 ± 4.0 |
| 4 MPa | 179.3 ± 3.1 | 156.1 ± 2.0 | 161.8 ± 3.6 | --- | --- | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciulla, M.; Canale, V.; Wolicki, R.D.; Pilato, S.; Bruni, P.; Ferrari, S.; Siani, G.; Fontana, A.; Di Profio, P. Enhanced CO2 Capture by Sorption on Electrospun Poly (Methyl Methacrylate). Separations 2023, 10, 505. https://doi.org/10.3390/separations10090505
Ciulla M, Canale V, Wolicki RD, Pilato S, Bruni P, Ferrari S, Siani G, Fontana A, Di Profio P. Enhanced CO2 Capture by Sorption on Electrospun Poly (Methyl Methacrylate). Separations. 2023; 10(9):505. https://doi.org/10.3390/separations10090505
Chicago/Turabian StyleCiulla, Michele, Valentino Canale, Rafal D. Wolicki, Serena Pilato, Pantaleone Bruni, Stefania Ferrari, Gabriella Siani, Antonella Fontana, and Pietro Di Profio. 2023. "Enhanced CO2 Capture by Sorption on Electrospun Poly (Methyl Methacrylate)" Separations 10, no. 9: 505. https://doi.org/10.3390/separations10090505
APA StyleCiulla, M., Canale, V., Wolicki, R. D., Pilato, S., Bruni, P., Ferrari, S., Siani, G., Fontana, A., & Di Profio, P. (2023). Enhanced CO2 Capture by Sorption on Electrospun Poly (Methyl Methacrylate). Separations, 10(9), 505. https://doi.org/10.3390/separations10090505