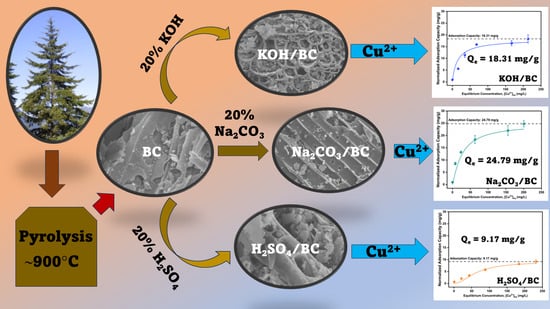
Comparison of Acid- and Base-Modified Biochar Derived from Douglas Fir for Removal of Copper (II) from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Douglas Fir Biochar
2.3. Modification of Douglas Fir Biochar (BC)
2.4. Characterization of Douglas Fir Biochar and Modified Douglas Fir Biochar
2.5. PH Point of Zero Charge (pHpzc)
2.6. Sorption of Copper onto BC, KOH/BC, Na2CO3/BC, and H2SO4/BC
2.6.1. Effect of Initial pH on Copper Sorption onto KOH/BC, Na2CO3/BC, and H2SO4/BC
2.6.2. Adsorption Kinetic
2.6.3. Adsorption Isotherm
2.6.4. Real Lake Water Sample Study
3. Results and Discussion
3.1. Surface Area, Pore Volume, and Pore Size Diameter
3.2. Surface Morphology and Elemental Compositions
3.3. XRD Analysis
3.4. FTIR Analysis
3.5. Thermogravimetric Analysis
3.6. The point of Zero Charge (pHpzc)
3.7. Effects of Initial Solution pH
3.8. Effect of Contact Time and Isotherm Study on Cu2+ Sorption onto BC, KOH/BC, Na2CO3/BC, and H2SO4/BC
3.9. Effect of Competitive Ions
3.10. Effect of Real Water Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sulaiman, F.R.; Hamzah, H.A. Heavy metals accumulation in suburban roadside plants of a tropical area (Jengka, Malaysia). Ecol. Process. 2018, 7, 28. [Google Scholar] [CrossRef]
- Boquete, M.T.; Lang, I.; Weidinger, M.; Richards, C.L.; Alonso, C. Patterns and mechanisms of heavy metal accumulation and tolerance in two terrestrial moss species with contrasting habitat specialization. Environ. Exp. Bot. 2021, 182, 104336. [Google Scholar] [CrossRef]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L.; Adams, W.J.; Menzie, C.A. Critical review of exposure and effects: Implications for setting regulatory health criteria for ingested copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.; Bonczek, R.; McGinn, C.; Land, M.; Bloom, L.; Sample, B.; Dolislager, F. The Risk Assessment Information System; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 1998. [Google Scholar]
- Shah, P.U.; Raval, N.P.; Shah, N.K. Adsorption of copper from an aqueous solution by chemically modified cassava starch. J. Mater. Environ. Sci. 2015, 6, 2573–2582. [Google Scholar]
- Kavitha, C.; Vijayasarathi, P.; Tamizhdurai, P.; Mythily, R.M.R.; Mangesh, V.L. Retraction notice to “Elimination of Lead by Biosorption on Parthenium stem powder using Box-Behnken Design”. S. Afr. J. Chem. Eng. 2022, 42, 270–279. [Google Scholar] [CrossRef]
- Râpă, M.; Ţurcanu, A.A.; Matei, E.; Predescu, A.M.; Pantilimon, M.C.; Coman, G.; Predescu, C. Adsorption of copper (II) from aqueous solutions with alginate/clay hybrid materials. Materials 2021, 14, 7187. [Google Scholar] [CrossRef]
- Martin, R. Lead and Copper Rule Revisions: A Case Study in Identifying and Tracking Lead Water Service Lines with ArcGIS Field Maps; The University of Arizona: Tucson, AZ, USA, 2022. [Google Scholar]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean. Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.; Summers, R.S.; Cook, S.M. Environmental comparison of biochar and activated carbon for tertiary wastewater treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar]
- Fuentes, A.L.B.; Arwenyo, B.; Nanney, A.L.; Ramirez, A.; Jamison, H.; Venson, B.; Mohan, D.; Mlsna, T.E.; Navarathna, C. Application of biochar for the removal of actinides and lanthanides from aqueous solutions. In Sustainable Biochar for Water and Wastewater Treatment, Elsevier: Amsterdam, The Netherlands, 2022; pp. 321–359.
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Najim, T.S.; Elais, N.J.; Dawood, A.A. Adsorption of copper and iron using low cost material as adsorbent. J. Chem. 2009, 6, 161–168. [Google Scholar] [CrossRef]
- Aydın, H.; Bulut, Y.; Yerlikaya, Ç. Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J. Environ. Manag. 2008, 87, 37–45. [Google Scholar] [CrossRef]
- Pavan Kumar, G.; Malla, K.A.; Yerra, B.; Srinivasa Rao, K. Removal of Cu (II) using three low-cost adsorbents and prediction of adsorption using artificial neural networks. Appl. Water Sci. 2019, 9, 44. [Google Scholar] [CrossRef]
- Smith, M.; Ha, S.; Amonette, J.E.; Dallmeyer, I.; Garcia-Perez, M. Enhancing cation exchange capacity of chars through ozonation. Biomass Bioenergy 2015, 81, 304–314. [Google Scholar] [CrossRef]
- Dewage, N.B.; Liyanage, A.S.; Pittman Jr, C.U.; Mohan, D.; Mlsna, T. Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar. Bioresour. Technol. 2018, 263, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.; Ricchetti, L.; Pittman Jr, C.U.; Anderson, R.; Mohan, D.; Mlsna, T. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem. Eng. J. 2018, 331, 480–491. [Google Scholar] [CrossRef]
- Rodrigo, P.M.; Navarathna, C.; Pham, M.T.; McClain, S.J.; Stokes, S.; Zhang, X.; Perez, F.; Gunatilake, S.R.; Karunanayake, A.G.; Anderson, R. Batch and fixed bed sorption of low to moderate concentrations of aqueous per-and poly-fluoroalkyl substances (PFAS) on Douglas fir biochar and its Fe3O4 hybrids. Chemosphere 2022, 308, 136155. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, T.N.; Nayanathara, R.O.; Navarathna, C.M.; Rodrigo, P.M.; Thirumalai, R.V.; Pittman Jr, C.U.; Kim, Y.; Mlsna, T.; Zhang, J.; Zhang, X. Pyrolytic synthesis of graphene-encapsulated zero-valent iron nanoparticles supported on biochar for heavy metal removal. Biochar 2022, 4, 70. [Google Scholar] [CrossRef]
- Chu, Y.; Zhu, S.; Wang, F.; Lei, W.; Xia, M.; Liao, C. Tyrosine-immobilized montmorillonite: An efficient adsorbent for removal of Pb2+ and Cu2+ from aqueous solution. J. Chem. Eng. Data 2019, 64, 3535–3546. [Google Scholar] [CrossRef]
- Karunaratne, T.N.; Rodrigo, P.M.; Oguntuyi, D.O.; Mlsna, T.E.; Zhang, J.; Zhang, X. Unraveling biochar surface area on structure and heavy metal removal performances of carbothermal reduced nanoscale zero-valent iron. J. Bioresour. Bioprod. 2023, 8, 388–398. [Google Scholar] [CrossRef]
- Aravamuthan, S.R.; Srinivasan, S.; Shukla, A.K. An in Situ Graphite-Grafted Alkaline Iron Electrode for Iron-Based Accumulators. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2013; p. 284. [Google Scholar]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Behazin, E.; Ogunsona, E.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M.; Anyia, A.O. Mechanical, chemical, and physical properties of wood and perennial grass biochars for possible composite application. BioResources 2016, 11, 1334–1348. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, N.; Xiao, Z.; Li, Z.; Zhang, D. Characterization of biochars derived from different materials and their effects on microbial dechlorination of pentachlorophenol in a consortium. RSC Adv. 2019, 9, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Siipola, V.; Tamminen, T.; Lahti, R.; Romar, H. Effects of Biomass Type, Carbonization Process, and Activated Carbons. Bioresources 2018, 13, 5976–6002. [Google Scholar] [CrossRef]
- Huang, H.; Tang, J.; Gao, K.; He, R.; Zhao, H.; Werner, D. Characterization of KOH modified biochars from different pyrolysis temperatures and enhanced adsorption of antibiotics. RSC Adv. 2017, 7, 14640–14648. [Google Scholar] [CrossRef]
- Rojas, R. Copper, lead and cadmium removal by Ca Al layered double hydroxides. Appl. Clay Sci. 2014, 87, 254–259. [Google Scholar] [CrossRef]










| Material a | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (Å) |
|---|---|---|---|
| BC | 578.90 ± 3.21 | 0.066 | 4.55 |
| KOH/BC | 389.30 ± 0.39 | 0.129 | 13.26 |
| H2SO4/BC | 326.65 ± 0.34 | 0.106 | 12.99 |
| Na2CO3/BC | 367.92 ± 3.44 | 0.127 | 13.76 |
| Material | C [%] | H [%] | O [%] | N [%] | S [%] | Ash [%] |
|---|---|---|---|---|---|---|
| BC | 92.29 ± 1.32 | 0.69 ± 0.01 | 4.75 | 0.13 ± 0.01 | 0.02 ± 0.00 | 2.13 ± 0.54 |
| KOH/BC | 81.62 ± 0.45 | 1.04 ± 0.03 | 10.98 | 0.2 ± 0.04 | 0.01 ± 0.01 | 6.15 ± 0.85 |
| H2SO4/BC | 76.39 ± 2.45 | 1.32 ± 0.05 | 16.08 | 0.25 ± 0.02 | 3.62 ± 0.80 | 1.89 ± 0.28 |
| Na2CO3/BC | 87.64 ± 0.87 | 0.83 ± 0.06 | 6.82 | 0.14 ± 0.07 | 0.05 ± 0.02 | 4.52 ± 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arwenyo, B.; Rodrigo, P.M.; Olabode, O.A.; Abeysinghe, H.P.; Tisdale, J.N.; Azuba, R.C.; Mlsna, T.E. Comparison of Acid- and Base-Modified Biochar Derived from Douglas Fir for Removal of Copper (II) from Wastewater. Separations 2024, 11, 78. https://doi.org/10.3390/separations11030078
Arwenyo B, Rodrigo PM, Olabode OA, Abeysinghe HP, Tisdale JN, Azuba RC, Mlsna TE. Comparison of Acid- and Base-Modified Biochar Derived from Douglas Fir for Removal of Copper (II) from Wastewater. Separations. 2024; 11(3):78. https://doi.org/10.3390/separations11030078
Chicago/Turabian StyleArwenyo, Beatrice, Prashan M. Rodrigo, Olalekan A. Olabode, Hashani P. Abeysinghe, Jessie N. Tisdale, Rose C. Azuba, and Todd E. Mlsna. 2024. "Comparison of Acid- and Base-Modified Biochar Derived from Douglas Fir for Removal of Copper (II) from Wastewater" Separations 11, no. 3: 78. https://doi.org/10.3390/separations11030078
APA StyleArwenyo, B., Rodrigo, P. M., Olabode, O. A., Abeysinghe, H. P., Tisdale, J. N., Azuba, R. C., & Mlsna, T. E. (2024). Comparison of Acid- and Base-Modified Biochar Derived from Douglas Fir for Removal of Copper (II) from Wastewater. Separations, 11(3), 78. https://doi.org/10.3390/separations11030078