Pomegranate Juice Clarification Using Ultrafiltration: Influence of the Type of Variety and Degree of Ripeness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Pomegranate Juice
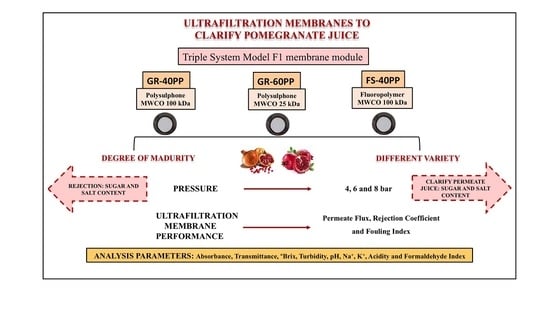
2.2. Membranes
2.3. Experimental Equipment
2.4. Experimental Procedure
2.4.1. Experimental Methods
2.4.2. Analytical Methods
3. Results and Discussion
3.1. Screening of the Optimal Membrane for Pomegranate Juice Clarification
3.2. Influence of the Chemical Composition of Membranes on Clarification
3.3. Influence of the Degree of Ripeness on Pomegranate Juice Clarification
3.4. Fouling Behavior of the Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. e-Libr. Evid. Nutr. Actions 2023. Available online: https://www.who.int/tools/elena/interventions/fruit-vegetables-ncds (accessed on 26 March 2024).
- Bagci, P.O. Effective Clarification of Pomegranate Juice: A Comparative Study of Pretreatment Methods and Their Influence on Ultrafiltration Flux. J. Food Eng. 2014, 141, 58–64. [Google Scholar] [CrossRef]
- Cheng, J.; Li, J.; Xiong, R.-G.; Wu, S.-X.; Huang, S.-Y.; Zhou, D.-D.; Saimaiti, A.; Shang, A.; Feng, Y.; Gan, R.-Y.; et al. Bioactive Compounds and Health Benefits of Pomegranate: An Updated Narrative Review. Food Biosci. 2023, 53, 102629. [Google Scholar] [CrossRef]
- EUROPEAN FRUIT JUICE ASSOCIATION (AIJN) Code Of Practice Reference Guidelines. Brussels (January 2024). Available online: https://aijn.eu/en/the-aijn-code-of-practice (accessed on 26 March 2024).
- Bassiri-Jahromi, S. Punica Granatum (Pomegranate) Activity in Health Promotion and Cancer Prevention. Oncol. Rev. 2018, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- García-Viguera, C.; Pérez-Vicente, A. La Granada. Alimento Rico En Polifenoles Antioxidantes y Bajo En Calorías. ANS Alimentación Nutrición y Salud 2004, 11, 113–120. [Google Scholar]
- Rojanathammanee, L.; Puig, K.L.; Combs, C.K. Pomegranate Polyphenols and Extract Inhibit Nuclear Factor of Activated T-Cell Activity and Microglial Activation in Vitro and in a Transgenic Mouse Model of Alzheimer Disease. J. Nutr. 2013, 143, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Cassano, A.; Rastogi, N.K. Advances in Membrane Technologies for Water Treatment Materials, Processes and Applications a Volume in Woodhead Publishing Series in Energy; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 978-1-78242-121-4. [Google Scholar]
- Cassano, A.; Conidi, C.; Drioli, E. Clarification and Concentration of Pomegranate Juice (Punica granatum L.) Using Membrane Processes. J. Food Eng. 2011, 107, 366–373. [Google Scholar] [CrossRef]
- Mirsaeedghazi, H.; Emam-Djomeh, Z.; Mousavi, S.M.; Ahmadkhaniha, R.; Shafiee, A. Effect of Membrane Clarification on the Physicochemical Properties of Pomegranate Juice. Int. J. Food Sci. Technol. 2010, 45, 1457–1463. [Google Scholar] [CrossRef]
- Vardin, H.; Fenercioğlu, H. Study on the Development of Pomegranate Juice Processing Technology: Clarification of Pomegranate Juice. Nahrung 2003, 47, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Drioli, E.; Cassano, A. Perspective of Membrane Technology in Pomegranate Juice Processing: A Review. Foods 2020, 9, 889. [Google Scholar] [CrossRef]
- Johanningsmeier, S.D.; Harris, G.K. Pomegranate as a Functional Food and Nutraceutical Source. Annu. Rev. Food Sci. Technol. 2011, 2, 181–201. [Google Scholar] [CrossRef] [PubMed]
- Surek, E.; Nilufer-Erdil, D. Changes in Phenolics and Antioxidant Activity at Each Step of Processing from Pomegranate into Nectar. Int. J. Food Sci. Nutr. 2014, 65, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and Purification of Phenolic Compounds from Pomegranate Juice by Ultrafiltration and Nanofiltration Membranes. J. Food Eng. 2017, 195, 1–13. [Google Scholar] [CrossRef]
- Baklouti, S.; Ellouze-Ghorbel, R.; Mokni, A. Chaabouni Clarification of Pomegranate Juice by Ultrafiltration: Study of Juice Quality and of the Fouling Mechanism. Fruits 2012, 67, 215–225. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A Mini-Review on Membrane Fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Urošević, T.; Povrenović, D.; Vukosavljević, P.; Urošević, I.; Stevanović, S. Recent Developments in Microfiltration and Ultrafiltration of Fruit Juices. Food Bioprod. Process. 2017, 106, 147–161. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; Gómez, E.; León, G.; Sánchez, A. Removal of Anilinic Compounds Using the NF-97 Membrane: Application of the Solution-Diffusion and SKK Models. Sep. Sci. Technol. 2016, 51, 2429–2439. [Google Scholar] [CrossRef]
- Mirsaeedghazi, H.; Emam-Djomeh, Z.; Mousavi, S.M.; Aroujalian, A.; Navidbakhsh, M. Clarification of Pomegranate Juice by Microfiltration with PVDF Membranes. Desalination 2010, 264, 243–248. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.C.; Lopéz-Cortés, I.; Salazar, D.M.; Ramalhosa, E.C.D. Physicochemical Changes and Antioxidant Activity of Juice, Skin, Pellicle and Seed of Pomegranate (Cv. Mollar de Elche) at Different Stages of Ripening. Food Technol. Biotechnol. 2015, 53, 397–406. [Google Scholar] [CrossRef]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.-E. Effect of in Vitro Gastrointestinal Digestion on Encapsulated and Nonencapsulated Phenolic Compounds of Carob (Ceratonia siliqua L.) Pulp Extracts and Their Antioxidant Capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef]
- Molaee Parvarei, M.; Khorshidian, N.; Yousefi, M.; Zendeboodi, F.; Mirsaeedghazi, H. Effect of Membrane Clarification on the Physicochemical Properties of Fruit Juices: A Review. Iran. J. Chem. Chem. Eng. 2022, 41, 3377–3390. [Google Scholar] [CrossRef]
- Mondal, S.; Cassano, A.; Conidi, C.; De, S. Quantification of Selective Transport of Fructose and Glucose During Membrane Filtration of Pomegranate Juice. Food Bioprocess Technol. 2021, 14, 272–286. [Google Scholar] [CrossRef]
- Morittu, V.M.; Mastellone, V.; Tundis, R.; Loizzo, M.R.; Tudisco, R.; Figoli, A.; Cassano, A.; Musco, N.; Britti, D.; Infascelli, F.; et al. Antioxidant, Biochemical, and In-Life Effects of Punica granatum L. Natural Juice vs. Clarified Juice by Polyvinylidene Fluoride Membrane. Foods 2020, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Al-Maiman, S.A.; Ahmad, D. Changes in Physical and Chemical Properties during Pomegranate (Punica granatum L.) Fruit Maturation. Food Chem. 2002, 76, 437–441. [Google Scholar] [CrossRef]
- Onsekizoglu, P. Production of High Quality Clarified Pomegranate Juice Concentrate by Membrane Processes. J. Memb. Sci. 2013, 442, 264–271. [Google Scholar] [CrossRef]




| Type of Membrane | GR-40PP | GR-60PP | FS-40PP |
|---|---|---|---|
| Manufacturer | Alfa Laval | Alfa Laval | Alfa Laval |
| Supporting material | Polypropylene | Polypropylene | Polypropylene |
| Composition | Polysulphone | Polysulphone | Fluoropolymer |
| Surface area (cm2) | 84.82 | 84.82 | 84.82 |
| Typical operating pressure (bar) | 1–10 | 1–10 | 1–10 |
| Tolerated pH range (at 25 °C) | 1–13 | 1–13 | 1–11 |
| Temperature range (°C) | 5–75 | 5–75 | 5–60 |
| MWCO * (kDa) | 100 | 25 | 100 |
| Pomegranate Mollar Juice | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | GR-60PP | GR-40PP | FS-40PP | ||||||
| Sfeed | Sperm | Sret | Sfeed | Sperm | Sret | Sfeed | Sperm | Sret | |
| ABS (420 nm) | 7.45 | 0.23 | 11.32 | 5.63 | 0.63 | 8.53 | 19.91 | 0.90 | 33.55 |
| %T (650 nm) | 0.33 | 100.80 | 1.23 | 5.88 | 91.20 | 1.53 | 0.95 | 26.10 | 0.61 |
| °Bx | 15.88 | 6.28 | 8.69 | 16.01 | 14.78 | 16.17 | 12.3 | 10.89 | 12.77 |
| Na+ (ppm) | 20 | 6 | 10 | 22 | 19 | 20 | 18 | 15 | 22 |
| K+ (ppm) | 1427 | 504 | 734 | 1386 | 1365 | 1407 | 1224 | 1345 | 1448 |
| Ph | 3.95 | 4.06 | 3.93 | 3.97 | 3.96 | 3.97 | 3.95 | 3.86 | 4.12 |
| %ACA | 0.27 | 0.07 | 0.16 | 0.27 | 0.21 | 0.28 | 0.22 | 0.20 | 0.24 |
| FI | 11.50 | 4.66 | 6.04 | 10.62 | 10.06 | 10.88 | 11.50 | 8.64 | 9.28 |
| Turbidity (NTU) | 2957 | 2.25 | 1940 | 578 | 1.02 | 1145 | 2882 | 83 | 4086 |
| Pomegranate Wonderful Juice | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | GR-60PP | GR-40PP | FS-40PP | ||||||
| Sfeed | Sperm | Sret | Sfeed | Sperm | Sret | Sfeed | Sperm | Sret | |
| ABS (420 nm) | 3.10 | 0.34 | 3.05 | 9.71 | 0.72 | 4.97 | 6.94 | 0.60 | 26.78 |
| %T (650 nm) | 1.30 | 100.60 | 31.60 | 0.40 | 57.00 | 0.20 | 1.27 | 3.71 | 0.01 |
| °Bx | 16.01 | 6.09 | 8.43 | 16.25 | 15.31 | 16.23 | 15.88 | 14.94 | 15.20 |
| Na+(ppm) | 15 | 8 | 6 | 26 | 20 | 26 | 27 | 22 | 26 |
| K+(ppm) | 2945 | 766 | 1244 | 2488 | 2250 | 1812 | 2772 | 2408 | 2448 |
| pH | 3.09 | 3.12 | 3.09 | 3.07 | 3.08 | 3.07 | 3.22 | 3.16 | 3.14 |
| %ACA | 1.45 | 0.51 | 0.74 | 1.45 | 1.36 | 1.48 | 1.56 | 1.44 | 1.60 |
| FI | - | 6.24 | 7.12 | 15.26 | 13.40 | 13.14 | 12.10 | 10.88 | 11.96 |
| Turbidity (NTU) | 1691 | 2.70 | 1641 | 2587 | 6.57 | 2339 | 814 | 371 | 4176 |
| Membrane | GR-60PP | GR-40PP | FS-40PP | |||||
|---|---|---|---|---|---|---|---|---|
| Permeate | Permeate | Permeate | ||||||
| Specifications | Min. | Max. | M | W | M | W | M | W |
| °Bx | 14.00 | - | 6.28 | 6.09 | 14.78 | 15.31 | 10.89 | 14.94 |
| Na+ (ppm) | - | 30 | 6 | 8 | 19 | 20 | 15 | 22 |
| K+ (ppm) | 1300 | 3000 | 504 | 766 | 1365 | 2250 | 1345 | 2408 |
| FI | 5.00 | 20.00 | 4.66 | 6.24 | 10.06 | 13.4 | 8.64 | 10.88 |
| Pomegranate Mollar Juice | Pomegranate Wonderful Juice | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Early | Late | Early | Late | ||||||||
| Sfeed | Sperm | Sret | Sfeed | Sperm | Sret | Sfeed | Sperm | Sret | Sfeed | Sperm | Sret | |
| ABS (420 nm) | 5.63 | 0.63 | 8.53 | 19.11 | 0.46 | 57.89 | 9.71 | 0.72 | 4.97 | 9.91 | 0.90 | 30.16 |
| %T (650 nm) | 5.88 | 91.2 | 1.53 | 3.00 | 36.5 | 0.04 | 0.40 | 57.00 | 0.20 | 1.50 | 54.50 | −0.02 |
| °Brix | 16.01 | 14.78 | 16.17 | 15.56 | 11.66 | 12.82 | 16.25 | 15.31 | 16.23 | 16.36 | 14.79 | 17.39 |
| Na+ (ppm) | 22 | 19 | 20 | 13 | 12 | 15 | 26 | 20 | 26 | 15 | 13 | 26 |
| K+ (ppm) | 1386 | 1365 | 1407 | 1417 | 1224 | 1365 | 2488 | 2250 | 1812 | 1724 | 2210 | 989 |
| pH | 3.97 | 3.96 | 3.97 | 4.45 | 4.49 | 4.43 | 3.07 | 3.08 | 3.07 | 3.16 | 3.53 | 3.50 |
| %ACA | 0.27 | 0.21 | 0.28 | 0.21 | 0.17 | 0.33 | 1.45 | 1.36 | 1.48 | 0.93 | 0.90 | 1.10 |
| FI | 10.62 | 10.06 | 10.88 | 14.22 | 17.06 | 11.96 | 15.26 | 13.40 | 13.14 | 18.72 | 23.94 | 20.20 |
| Turbidity (NTU) | 578 | 1.02 | 1145 | 901 | 31.2 | 5440 | 2587 | 6.57 | 2339 | 500 | 12.80 | 4433 |
| MOLLAR | WONDERFUL | |||||
|---|---|---|---|---|---|---|
| Specifications | Min. | Max. | Early | Late | Early | Late |
| °Bx | 14 | - | 14.78 | 11.66 | 15.31 | 14.79 |
| Na+ (ppm) | - | 30 | 19 | 12 | 20 | 13 |
| K+ (ppm) | 1300 | 3000 | 1365 | 1224 | 2250 | 2210 |
| FI | 5.00 | 20.00 | 10.06 | 17.06 | 13.4 | 23.94 |
| Pressure Range (bar) | |||
|---|---|---|---|
| Membranes | [4.4–5.75] | [5.75–6.5] | [6.5–9.2] |
| GR-60PP | 89.50 | 83.11 | 99.14 |
| GR-40PP1 | 57.12 | 54.78 | 57.80 |
| FS-40PP | 81.57 | 85.12 | 85.34 |
| GR-40PP2 | 76.54 | 78.88 | 80.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, A.M.; Macario, J.A.; Abellán-Baeza, M.; Sánchez-Moya, T.; López-Nicolás, R.; Marín-Iniesta, F. Pomegranate Juice Clarification Using Ultrafiltration: Influence of the Type of Variety and Degree of Ripeness. Separations 2024, 11, 134. https://doi.org/10.3390/separations11050134
Hidalgo AM, Macario JA, Abellán-Baeza M, Sánchez-Moya T, López-Nicolás R, Marín-Iniesta F. Pomegranate Juice Clarification Using Ultrafiltration: Influence of the Type of Variety and Degree of Ripeness. Separations. 2024; 11(5):134. https://doi.org/10.3390/separations11050134
Chicago/Turabian StyleHidalgo, Asunción M., José A. Macario, Marta Abellán-Baeza, Teresa Sánchez-Moya, Rubén López-Nicolás, and Fulgencio Marín-Iniesta. 2024. "Pomegranate Juice Clarification Using Ultrafiltration: Influence of the Type of Variety and Degree of Ripeness" Separations 11, no. 5: 134. https://doi.org/10.3390/separations11050134
APA StyleHidalgo, A. M., Macario, J. A., Abellán-Baeza, M., Sánchez-Moya, T., López-Nicolás, R., & Marín-Iniesta, F. (2024). Pomegranate Juice Clarification Using Ultrafiltration: Influence of the Type of Variety and Degree of Ripeness. Separations, 11(5), 134. https://doi.org/10.3390/separations11050134